Draft Calls for Screening Those at Risk for Latent TB - AAFP News
The draft gives a "B" recommendation to screen asymptomatic adults who are at increased risk, such as those who have suppressed immune systems or are taking immunosuppressive medications, or who were born in or previously lived in countries with an increased prevalence of TB. It does not apply to adults with symptoms of TB or to people younger than 18.
Overall, the draft is consistent with the task force's 2016 recommendation, which also received a "B" grade.
In 2020, the last year for which data is available, more than 71% of all cases of active TB in the United States occurred among people born outside the United States. Compared with active TB, in which patients show clear signs of illness and may be infectious, people with latent TB do not have symptoms or feel sick, and cannot spread TB bacteria to others. While many people with latent TB infection never develop TB, others can become ill weeks or even years after becoming infected.
Screening and Treatment
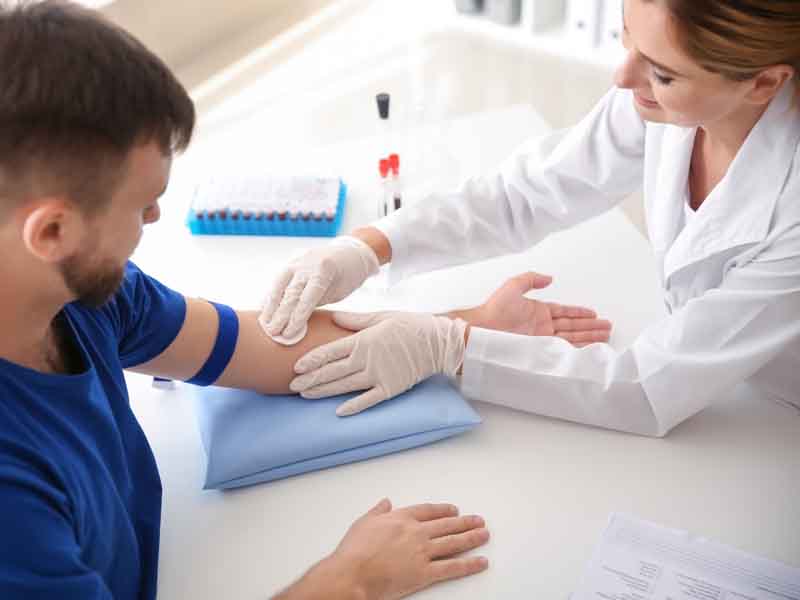
Two types of screening tests for latent TB infection are available in the United States: the tuberculin skin test and the interferon-gamma release assay. Both tests are moderately sensitive and highly specific.
Each type has advantages and drawbacks. Interferon-gamma release assay screening can be performed in one office visit with a single venous blood sample; however, samples must be delivered to a laboratory within a specific timeframe. The tuberculin skin test is somewhat less invasive but requires patients to return for a follow-up visit 48 to 72 hours later.
The task force found no studies that directly reported on the benefits or harms of screening. In addition, while the task force found no evidence on the ideal frequency of screening, it suggested "a reasonable approach" of repeat screening based on specific risk factors.
Several types of antibiotics are available for the treatment of latent TB infection, including both short- and long-course regimens, although some concerns were expressed about isoniazid, which may cause liver damage. The task force directed clinicians to the latest treatment recommendations from the CDC and the National Tuberculosis Controllers Association.
Further Research and Resources
Due to a small number of evidence gaps, the task force called for more research on identifying those at increased risk for latent TB infection and on repeat screening and screening strategies for certain populations.
The task force also suggested resources for clinicians, including
Comments
Post a Comment