“Diagnosis and management of acute respiratory distress syndrome - CMAJ” plus 2 more
“Diagnosis and management of acute respiratory distress syndrome - CMAJ” plus 2 more |
- Diagnosis and management of acute respiratory distress syndrome - CMAJ
- Underlying illness, respiratory infection raise risk for severe COVID in kids - CIDRAP
- COPD lung sounds: Types, descriptions, treatment, and more - Medical News Today
| Diagnosis and management of acute respiratory distress syndrome - CMAJ Posted: 25 May 2021 12:00 AM PDT KEY POINTS
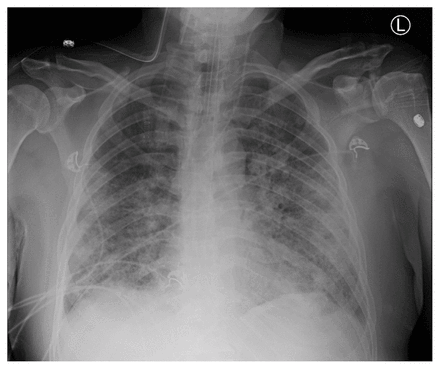
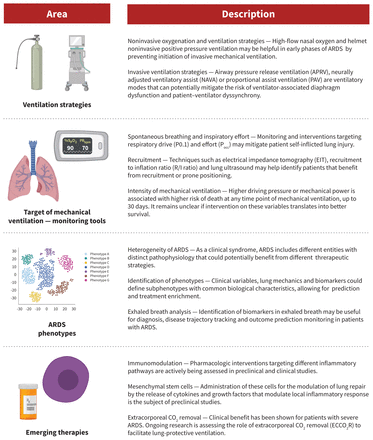
Acute respiratory distress syndrome (ARDS) is a life-threatening form of respiratory failure, characterized by acute, diffuse, inflammatory lung injury,1 that results in increased alveolar capillary permeability and the development of nonhydrostatic pulmonary edema. Clinically, ARDS manifests as marked hypoxemia and respiratory distress; patients often progress to respiratory failure that requires invasive mechanical ventilation in the intensive care unit (ICU). The risk of death is high. A variety of conditions can cause ARDS, including pneumonia, extrapulmonary sepsis or septic shock, trauma and pancreatitis. Despite consensus guidelines on the management of ARDS,2–4 substantial worldwide variation in management continues, and gaps in evidence remain, including in the context of ARDS associated with COVID-19.5,6 We provide an update on the diagnosis and management of ARDS for the generalist clinician, based on recent clinical practice guidelines, systematic reviews and original studies (Box 1). Box 1: Evidence used in this reviewWe conducted a targeted search of MEDLINE, Embase and the Cochrane Database of Systematic Reviews, from inception to Feb. 20, 2021, for randomized controlled trials, scoping reviews, systematic reviews, meta-analyses and clinical practice guidelines. We searched for the terms "acute respiratory distress syndrome," "ARDS," "respiratory failure" and "acute lung injury." We further enhanced our search by evaluating the reference lists of selected articles and supplemented our search with literature from our own collections. What is ARDS and how is it diagnosed?Acute respiratory distress syndrome was originally described in 1967 as a clinical syndrome characterized by acute onset of tachypnea, hypoxemia and loss of lung compliance after a variety of stimuli; the original description also noted that ARDS was not responsive to usual and ordinary methods of respiratory therapy.7 The hallmark of this syndrome is diffuse lung inflammation, resulting in development of pulmonary edema. Morphologically, the acute phase of ARDS is characterized by diffuse alveolar damage.1 Formal diagnostic criteria for ARDS were not widely accepted until the 1994 American–European Consensus Conference (AECC).8 The AECC criteria include the acute onset of hypoxemia, the presence of noncardiogenic, bilateral infiltrates on chest radiographs and the absence of left atrial hypertension. The presence of hypoxemia was quantified using the ratio of partial pressure of arterial oxygen and the fraction of inspired oxygen (Pao2/FiO2), with a Pao2/FiO2 < 200 mm Hg required for diagnosis of ARDS. The AECC definition was limited by several factors, namely the lack of an explicit time of onset, potential interobserver variability of the chest radiograph and the requirement for pulmonary artery catheterization to rule out left atrial hypertension.1 In 2012, the clinical criteria for diagnosis of ARDS were refined to address these limitations, resulting in the Berlin definition.9 For diagnosis of ARDS, the patient must have new or worsening symptoms within 1 week of a known clinical insult; bilateral opacities observable on anteroposterior chest radiographs that are not due to effusions, nodules or lobar or lung collapse; and hypoxemia, defined by a Pao2/FiO2 < 300 mm Hg and a minimum positive end-expiratory pressure ≥ 5 cm H2O, that is not fully explained by cardiac failure or fluid overload (Figure 1). Figure 1: Anteroposterior chest radiograph showing bilateral pulmonary infiltrates, consist with acute respiratory distress syndrome. The Berlin definition also identified mutually exclusive categories of ARDS severity based on the degree of hypoxemia, including mild (Pao2/FiO2 200–300 mm Hg), moderate (Pao2/FiO2 100–200 mm Hg) and severe (Pao2/FiO2 < 100 mm Hg) ARDS. These categories correspond to prognosis, with higher severity associated with increased mortality rates in the data sets used for derivation of the criteria.9,10 What is the burden of ARDS?In the LUNG SAFE prospective cohort study, the Berlin definition was used to identify patients with ARDS from 459 ICUs in 50 countries across 5 continents.5 In this study, ARDS accounted for 10.4% of all ICU admissions and 23.4% of patients requiring mechanical ventilation. The most common causes of ARDS were pneumonia, extrapulmonary sepsis, aspiration and trauma. The median duration of mechanical ventilation of patients with ARDS was 8 (interquartile range 4–16) days. The number of deaths was substantial, with 39.6% of patients dying in hospital, and increased with severity of ARDS (34.9%, 40.3% and 46.1% of patients with mild, moderate and severe disease dying in hospital, respectively). Administrative data show that, although mortality rates from ARDS have decreased overall in the last 2 decades, racial and sex disparities still exist and survivors have substantial morbidity.11 Survivors of ARDS show severe muscle weakness and fatigue that persist up to 5 years after hospital discharge, which result in impaired functional ability and reduced exercise tolerance.12,13 Furthermore, survivors describe important psychological, cognitive and economic sequelae associated with ARDS.13 What are the mainstays of treatment?The LUNG SAFE study found variations in the use of evidence-based treatments for ARDS across centres in Europe.5 Few therapies are based on strong evidence, but over the past 2 decades, important advances have been made in the management of the syndrome, particularly related to ventilation. These have subsequently been incorporated into clinical practice guidelines.2–4 Figure 2 summarizes a suggested approach to the management of patients with ARDS. Figure 2: Suggested treatment algorithm showing risk stratification and tiered approach to therapy for patients with acute respiratory distress syndrome (ARDS). Note: HFNC = high-flow nasal cannula, HFOV = high-frequency oscillatory ventilation, PEEP = positive end-expiratory pressure, PBW = predicted body weight, VV-ECMO = venovenous extracorporeal membrane oxygenation. Mechanical ventilationLung-protective mechanical ventilation is the cornerstone of ARDS therapy. The recommendations of recent clinical practice guidelines relating to mechanical ventilation are summarized in Table 1. The predominant goal is avoidance of ventilator-induced lung injury, an iatrogenic form of lung injury that worsens inflammation and is associated with worse outcomes in patients who are mechanically ventilated.30 Ventilator-induced lung injury occurs when excessive mechanical stress (e.g., large tidal volume) is translated into an inflammatory response (i.e., volutrauma) that can propagate through the circulation and lead to distant organ failure (i.e., biotrauma). Randomized trials have shown that ventilation with lower tidal volumes relative to predicted body weight and limiting plateau pressures resulted in substantially improved mortality rates among patients with ARDS.15,16 Lung-protective ventilation comes at the cost of possible hypercapnia and resultant acidosis, which may be tolerated if it is not severe.16 View this table: Table 1: Summary of mechanical ventilation interventions for the acute respiratory distress syndrome (ARDS) and recommendations from the clinical practice guidelines of the American Thoracic Society (ATS), European Society of Intensive Care Medicine (ESICM), Society of Critical Care Medicine (SCCM), Societé de réanimation de langue Française (SRLF) and Intensive Care Society (ICS) Existing guidelines suggest consideration of higher levels of positive end-expiratory pressure in patients with moderate-to-severe ARDS.2–4 Maintaining higher positive end-expiratory pressure has the potential advantage of minimizing cyclical alveolar collapse and subsequent shearing injury to the lungs. However, excess positive end-expiratory pressure may also impair hemodynamics and lead to lung overdistention. This therapy has been shown to be efficacious only in patients with moderate-to-severe ARDS.17 Other methods to improve ventilation, such as high-frequency oscillatory ventilation have not been found to be efficacious22,23 and guidelines have recommended against the routine use of high-frequency oscillatory ventilation in patients with ARDS.2–4 Noninvasive ventilation may be considered in patients with mild ARDS, but is unlikely to be beneficial in patients with more severe disease.14 In a recent meta-analysis, high-flow nasal cannula oxygen therapy was shown to reduce the need for intubation and mechanical ventilation in patients with acute hypoxemic respiratory failure, but not to reduce mortality rates.31 Prone positioningThe incidence of ventilator-induced lung injury may be reduced by placing patients in the prone position. Mechanical ventilation in the supine position can result in atelectasis and derecruitment of the most dependent lung regions. Prone positioning redistributes mechanical forces through the injured lung, resulting in more homogeneous lung inflation and recruitment of alveoli in the dependent lung regions. In patients with ARDS and a Pao2/FiO2 < 150 mm Hg, high-quality evidence shows that prone positioning reduces the risk of death without an increase in serious complications.18 Therefore, use of routine prone positioning in patients with severe ARDS is recommended by guidelines.2–4 Extracorporeal life supportThe use of venovenous extracorporeal membrane oxygenation (VV-ECMO) has emerged as a viable treatment option for patients with severe ARDS, Previously considered a rescue therapy for refractory ARDS, sufficient evidence now exists regarding the efficacy of VV-ECMO in patients with severe ARDS who are deteriorating despite other therapies being optimized.27–29 For these patients, VV-ECMO can act as a bridge to recovery. Deoxygenated blood is diverted via cannulae from the systemic circulation to an extracorporeal membrane lung that oxygenates and clears carbon dioxide from the blood, and returns the oxygenated blood to circulation. The use of such extracorporeal gas exchange support allows for the use of lower ventilatory pressures to the injured lung, minimizing ventilator-induced lung injury in severely ill patients. As ECMO is a valid treatment, clinicians should discuss potential cases with ECMO referral centres early in a patient's disease course rather than as a last resort. Pharmacologic therapyCorticosteroids have been much studied as a pharmacological therapy for ARDS. Theoretically, they act to decrease overall lung inflammation in ARDS, and may reduce the risk of death in severe ARDS.24 However, the use of corticosteroids in critically ill patients is also associated with important adverse events, including hypernatremia, hyperglycemia and neuromuscular weakness. The latter can be devastating to patients with ARDS, and clinicians must consider and weigh these potential risks. Adjunctive therapy with neuromuscular blockade and associated deep sedation may also be considered for patients with ARDS receiving mechanical ventilation. Delivery of regular, low tidal volumes may be difficult in the patient who is awake and spontaneously breathing (and often tachypneic), a situation referred to as patient–ventilator dysynchrony.32 Therefore, deep sedation and neuromuscular blockade have been trialled in combination with mechanical ventilation for severe ARDS. Findings from studies of early use of neuromuscular blockade in patients with ARDS are conflicting.19,20 However, it may be considered for optimization of oxygenation and ventilation, if not recommended as a routine intervention, in all patients with moderate-to-severe ARDS.21 Other pharmacologic therapies for ARDS have also been trialled, with various degrees of success. Maintaining a conservative fluid balance in tandem with use of diuretics has been shown to reduce the duration of mechanical ventilation and improve lung function in patients with ARDS, and should be considered routinely.33 Inhaled nitric oxide may theoretically reduce pulmonary vascular resistance and ventilation–perfusion mismatch, although randomized data do not support a mortality benefit, and may in fact suggest harm.25 Finally, the use of aerosolized prostacyclin for ARDS has been studied,26 but further study of its effects is required before it could be recommended for routine use. Is ARDS that is associated with COVID-19 a distinct entity?The COVID-19 pandemic brought the management of ARDS into the spotlight in 2020. Development of ARDS secondary to severe COVID-19 was (and is) common, and it was unclear whether COVID-19–associated ARDS was a distinct entity from other forms of ARDS, and whether a different management strategy was necessary.34 Consideration of alternate strategies for COVID-19–associated ARDS arose from early reports.35 Two distinct ARDS phenotypes were described among patients with COVID-19: type H, marked by high pulmonary elastance, high ventilation/perfusion ratio, high lung weight, and high alveolar recruitability (consistent with typical severe ARDS), and the more novel type L, marked by low values for the same variables.36 Some experts suggested that most patients with COVID-19–associated ARDS would initially present with type L characteristics, with only some transitioning to type H, and asserted that clinicians should therefore consider early intubation in patients with type L ARDS, further suggesting that these patients might tolerate higher tidal volumes without risk of ventilator-induced lung injury.35,36 However, accumulating evidence does not support this characterization of COVID-19–associated ARDS.34 First, patients without COVID-19 who meet the Berlin definition of ARDS are known to have variable degrees of pulmonary elastance and recruitability,5 and the idea of distinct phenotypes among patients with ARDS has been previously proposed and shown in a more rigorous fashion.37 Despite this, identification of phenotypes has not yet translated into differences in the management of ARDS by clinicians.34 Second, further reports evaluating the lung mechanics of patients with COVID-19–associated ARDS show that these patients are similar to conventional patients with ARDS.38 In fact, the proposed phenotypes of COVID-19–associated ARDS likely represent the natural evolution of ARDS. As such, using existing evidence-based therapies that protect the lungs and avoid iatrogenic injury likely represents the best way forward.39 Although future evidence may change management approaches, no convincing evidence currently suggests that COVID-19–associated ARDS is a distinct entity, or that an alternative treatment strategy is necessary, particularly with regard to ventilation. In fact, therapies that are commonly used for the treatment of ARDS may be effective for COVID-19–associated ARDS. Patients with COVID-19 may benefit from noninvasive ventilation (namely high-flow nasal cannula) and prone positioning while awake, both of which appear to improve hypoxemia and avoid intubation,40,41 and are the subject of ongoing randomized trials. Most notable is the use of steroids (primarily dexamethasone), which has been shown to reduce mortality rates among mechanically ventilated patients with COVID-19.42,43 Although the use of tocilizumab, a monoclonal antibody, may be effective in reducing mechanical ventilation and death in hospitalized patients with COVID-19,44,45 the use of therapeutic anticoagulation among patients with severe COVID-19 does not appear to be beneficial.46 Finally, many patients with ARDS, whether associated with COVID-19 or not, may require a lengthy duration of mechanical ventilation. Therefore, tracheostomy may become necessary, and clinicians should adhere to recommendations related to safety, conduct and management of tracheostomy.47 What uncertainties remain regarding the management of ARDS?Important areas for future study in ARDS therapy are summarized in Figure 3. Although lung-protective ventilation with traditional pressure- or volume-targeted modes have been the cornerstone of ARDS management, novel ventilatory modes may also be efficacious. First, airway pressure release ventilation (APRV) is a pressure-control mode of ventilation that may minimize ventilator-induced lung injury. This approach periodically deflates the lungs ("release") from a higher level of continuous positive airway pressure, rather than trying to inflate the lung to ideal lung volumes by overcoming poor compliance with higher pressures. Theoretically, by maintaining continuous pressures at moderate levels, APRV may reduce ventilator-induced lung injury. However, a recent randomized trial found that APRV had no impact on mortality rates in ARDS, although it was associated with reduced duration of mechanical ventilation and ICU length of stay, compared with volume-controlled, lung-protective ventilation.48 Further clinical trials on the efficacy of APRV are needed. The potential for exacerbation of lung injury through patient self-inflicted lung injury (P-SILI) is another interesting area of study. Although supported by a strong physiologic rationale, there is a paucity of human data to understand P-SILI.49 Theoretically, the risk of P-SILI may be mitigated by controlling respiratory drive and effort through neuromuscular blockade, sedation or extracorporeal life support. At present, there is limited evidence that controlling respiratory effort and drive is associated with improved outcomes in patients with ARDS.50 Finally, although VV-ECMO is beneficial for patients with severe ARDS for whom conventional management is failing, another emerging form of extracorporeal life support that may be valuable in the management of moderate-to-severe ARDS is extracorporeal carbon dioxide removal.51 For patients receiving mechanical ventilation with very low tidal volumes, there is a risk of hypoventilation and resultant hypercapnea and acidosis. Extracorporeal carbon dioxide removal can facilitate very low tidal volumes by providing an extracorporeal method to reduce carbon dioxide. Unlike VV-ECMO, this approach uses smaller catheters, though there are important associated risks, mainly related to bleeding.52 Extracorporeal carbon dioxide removal may be considered for patients with moderate ARDS and may prevent progression to severe disease; trials to study its efficacy are ongoing. ConclusionAcute respiratory distress syndrome causes respiratory failure that most commonly occurs secondary to pneumonia, sepsis, trauma or aspiration. Increasing severity of hypoxemia in ARDS is associated with high risk of mortality. Management of ARDS is largely focused on supportive management, lung-protective ventilation and minimizing iatrogenic forms of lung injury, with extracorporeal life support as an option for patients who continue to deteriorate despite these supportive therapies. Acute respiratory distress syndrome that is associated with COVID-19 does not appear to be distinct from the conventional syndrome, and existing therapies should remain the mainstay of treatment. Footnotes
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/ |
| Underlying illness, respiratory infection raise risk for severe COVID in kids - CIDRAP Posted: 07 Jun 2021 01:34 PM PDT  Of two new studies on severe COVID-19 in children, the first finds that more than one in four had an underlying medical condition, and the second shows that kids diagnosed as having acute lower respiratory tract infection (ALRI) were over twice as likely to require invasive mechanical ventilation. Type 1 diabetes, obesity, heart problemsIn the first study, published today in JAMA Network Open, researchers from the US Centers for Disease Control and Prevention used the Premier Healthcare Database Special COVID-19 Release, which houses data from 872 hospitals, to study 43,465 patients 18 and younger who visited an emergency department or were hospitalized for severe COVID-19 from March 2020 to January 2021. They found that 28.7% of all COVID-19 patients had at least one chronic condition, the most common of which were asthma (10.2%), neurodevelopmental disorders (3.9%), anxiety and fear-related conditions (3.2%), depression (2.8%), and obesity (2.5%). Underlying illness were identified in 62.9% of hospitalized COVID-19 patients. The strongest risk factors for hospitalization included type 1 diabetes (adjusted risk ratio [aRR], 4.60) and obesity (aRR, 3.07), while type 1 diabetes (aRR, 2.38) and cardiac and circulatory congenital disorders (aRR, 1.72) were the strongest risks for severe illness. Prioritizing public health, vaccination effortsAmong children younger than 2 years, prematurity was a risk factor for severe COVID-19 disease (aRR, 1.83). Chronic and complex chronic illnesses were risk factors for hospitalization (aRRs, 2.91 and 7.86, respectively) and serious illness (aRRs,1.95 and 2.86). Chronic conditions tied to a higher risk of COVID-19 hospitalization of children of any age were epilepsy and/or convulsions (aRR, 1.97); dependence on medical support, such as a feeding tube (aRR, 1.96); trauma and stress-related disorders (aRR, 1.82); neurodevelopmental disorders (aRR, 1.64); type 2 diabetes (aRR, 1.59); depression (aRR, 1.58); essential high blood pressure (aRR, 1.51); anxiety and fear-related disorders (aRR, 1.47); and asthma (aRR, 1.23). Among the 4,302 children admitted to a hospital with COVID-19, risk intensive care unit (ICU) admission, mechanical ventilation, or death was highest in those with type 1 diabetes (aRR, 2.38), cardiac and circulatory congenital disorders (aRR, 1.72), and epilepsy and/or convulsions (aRR, 1.71). Other conditions linked to higher risk of poor outcomes included obesity (aRR, 1.42); essential high blood pressure (aRR, 1.39); sleep disorders such as sleep apnea (aRR, 1.26); and dependence on medical support (aRR, 1.25). Of all COVID-19 patients, 29.6% were admitted to the ICU, 9.9% were hospitalized, 6.4% required invasive mechanical ventilation, and 0.9% died. Median patient age was 12 years (range, 4 to 16), 52.8% were female, 33.0% were Hispanic or Latino, and 24.1% were Black. The researchers said that public health mitigation efforts and vaccine prioritization should be considered for children with chronic underlying diseases. "Health care practitioners can consider the potential need for cautious clinical management of children with these conditions and COVID-19," they concluded. "Further epidemiologic investigation could provide insight into the causal pathways underlying our findings and identify other factors that place children at increased risk of severe COVID-19 illness." Death, need for mechanical ventilationIn a letter to the editor late last week in the Journal of Infection, Vivian Botelho Lorenzo, MD, and Cristiana Nascimento-Carvalho, MD, PhD, both of the Federal University of Bahia School of Medicine in Salvador, Brazil, described a retrospective study of 210 children younger than 17 years admitted to a pediatric ICU with severe ALRI from April 2020 to April 2021. The median age was 2.8 years. Of the 210 ALRI patients, 29.5% tested positive for COVID-19, 15.7% needed mechanical ventilation, and 3.8% died. COVID-19 patients were more likely than their peers to need mechanical ventilation (25.8% vs 11.5%) and to die (8.1% vs 2.0%). Boys and patients with sickle cell disease were more likely than others to test positive for COVID-19 (67.7% vs 52.7%; 6.5% vs 0%). Among all patients, the most common ALRI symptoms were shortness of breath (98.1%), cough (68.6%), and fever (57.6%), while the most common signs were oxygen concentration below 92% (73.3%), needing respiratory support (62.4%), and dehydration (39.5%). The researchers note that wheezing at admission was more likely to be a symptom in those who weren't admitted (21.0% vs 38.5%, respectively). More than one in three patients also had an underlying medical condition (34.3%), the most common of which were chronic lung disease (18.6%), neurological diseases other than epilepsy (7.1%), and epilepsy (5.2%). Also, three of the five COVID-19 patients who died had an underlying condition, with two diagnosed as having cerebral palsy and one having floppy infant syndrome. The researchers noted that amid the coronavirus pandemic, about two-thirds of patients were hospitalized with severe ALRIs without COVID-19. "In 2016, 3 years before the beginning of the current COVID-19 pandemic, ALRI was estimated to cause 652,572 deaths among children younger than 5 years all over the world," they wrote. "Therefore, besides SARS-CoV-2, other causative agents remain causing severe ALRI." The authors said that clinicians should be aware that children with severe ALRI and COVID-19 need mechanical ventilation more often than other patients and that children with sickle cell disease should be prioritized for COVID-19 vaccination. |
| COPD lung sounds: Types, descriptions, treatment, and more - Medical News Today Posted: 13 May 2021 09:13 AM PDT  Chronic obstructive pulmonary disease (COPD) refers to a group of lung conditions that cause breathing-related difficulties. It can give rise to several different lung sounds. COPD can cause a variety of different lung sounds, including rhonchi, wheezing, and crackling. This article will discuss the types of lung sounds that can occur alongside COPD. It will also look at how healthy breathing sounds and how doctors diagnose COPD. The National Heart, Lung, and Blood Institute notes that COPD can cause a wheezing sound when a person breathes. Wheezing sounds occur due to the vibrations of the narrowed walls of small airways. COPD causes the small airways to narrow, resulting in whistling sounds as air attempts to travel through the narrow passages during exhalation. When wheezing is present in the lungs, it manifests as a high pitched whistling sound during expiration. Crackling, otherwise known as rales, describes a sound in the lungs that resembles a crackling or clicking sound when a person breathes in. According to one 2021 study, crackling sounds are common in COPD. There are two distinct types of crackling sounds detectable in the lungs: coarse and fine. Coarse crackles are more typical of COPD and present as prolonged, low pitched sounds. Fine crackles are more high pitched. The crackling noise stems from air bubbles passing through fluid, such as mucus, in the airways. Coughing occurs as a biological reaction to clear this fluid. According to one 2018 article, rhonchi are low pitched, continuous gurgling or bubbling sounds that a person might hear when they inhale and exhale. These breathing sounds point to a buildup of secretions in the upper airways, which can occur with COPD. Generally, healthy breathing sounds are silent through the mouth. Doctors may classify normal breathing sounds from the lungs as:
A doctor can evaluate these sounds most effectively with a stethoscope, and they will analyze all sounds based on their duration, intensity, pitch, and timing. Healthy lungs produce sounds emanating from their respective regions. The most common test for diagnosing COPD is spirometry. This measures how much air a person can breathe in and out of the lungs. It also measures the speed at which a person exhales or completely empties the lungs. Other tests for COPD may involve chest X-rays or arterial blood gas tests to measure oxygenation in the blood. This latter test will reveal how efficiently the lungs disperse oxygen throughout the body. Although there is currently no curative treatment available for COPD, there are many treatment options to help relieve the symptoms and reduce the risk of flare-ups. Some possible treatments include:
A person can also try complementary therapies, such as yoga, massage, and acupuncture. Although these will not treat COPD, they may be able to improve the symptoms. A person should always discuss their treatment options with a doctor. Some other symptoms of COPD may include:
Severe COPD can cause:
A person should contact a doctor if they notice any symptoms of COPD. They should seek emergency care if they experience:
A person should also seek immediate help if their recommended treatment is not working. It is important to contact a doctor any time breathing is difficult or becomes laborious for prolonged periods of time. COPD can cause a person to experience a variety of lung sounds, which typically include:
If a person notices any of the symptoms of COPD, they should contact a doctor. |
| You are subscribed to email updates from "respiratory depression,respiratory depression symptoms,respiratory depression treatment" - Google News. To stop receiving these emails, you may unsubscribe now. | Email delivery powered by Google |
| Google, 1600 Amphitheatre Parkway, Mountain View, CA 94043, United States | |

Comments
Post a Comment