“A Runner Suddenly Developed Asthma. It Was Stranger Than It Seemed. - The New York Times” plus 1 more
“A Runner Suddenly Developed Asthma. It Was Stranger Than It Seemed. - The New York Times” plus 1 more |
| A Runner Suddenly Developed Asthma. It Was Stranger Than It Seemed. - The New York Times Posted: 12 Dec 2019 11:25 AM PST  It was chest pain that brought the 34-year-old woman to the emergency room at Montefiore Medical Center in the Bronx. She'd been coughing for days, but that morning the pain was so bad she was worried that it had turned into pneumonia. She tried to tough it out, but when it was no better by the end of the day, she decided to go to the E.R. It took a few hours, but finally the physician assistant caring for her in the E.R. brought some good news. She didn't have pneumonia; she didn't have a clot in her lungs. This was just muscle strain from coughing. The year before, she was told she had asthma. She was given inhalers to stop the coughing and wheezing, but they didn't seem to do much. So she didn't use them. The P.A. encouraged the woman to use her inhalers; they really would prevent the episodes of coughing and wheezing that had sent her to the E.R. so often that year. The woman nodded; she'd heard this speech before. The P.A. asked whether she'd like ibuprofen for the pain. Absolutely not, the patient said. After years of taking it for muscle pain after working out or racing, she had developed some kind of allergy to it. What about ketorolac? the P.A. offered. It's the same type of painkiller but a completely different compound. Pain relief sounded appealing. But within seconds of getting the intravenous medication, the woman felt an intense pressure in her chest. Her windpipe narrowed as if she had something stuck in her throat. She tried to shout, "I can't breathe!" The only sound that came out was an unintelligible whisper. The P.A.'s face turned pale, and she helped the struggling woman onto a stretcher and clapped a mask hissing with aerosolized medications over her nose and mouth. She'd had these treatments when her asthma flared, and they'd always helped. Not this time. Furious with fear, she tore off the mask and lunged at the young woman. The cubicle quickly filled with people who looked worried. And then everything went black. Healthy and Then NotThe woman's mother was shocked by the rapidly unfolding events. Her daughter had been healthy and active her whole life. She had competed in triathlons and endurance races called Tough Mudders. But the past year, she told the doctors in the intensive-care unit, where her daughter was lying unconscious with a thick breathing tube down her throat, everything changed. Her daughter never had asthma until recently. Twice in the past month, she sounded so short of breath on the phone that the mother insisted she go to the emergency room. Each time, they gave her some prednisone and a breathing treatment. She'd get better, and they'd send her home. [Read about the risks of combining vigorous exercise and painkillers.] At the hospital this time, it was three days before the doctors were able to stop the sedating medications and remove the breathing tube. Once awake, the patient kept asking how this could have happened. She'd never had asthma. She'd never been allergic to anything. But now she had severe asthma and was allergic to not one but two painkillers. The doctors in the hospital had no answers and suggested she see an allergist. Image  A True Allergic Reaction?The patient went to see Dr. Elina Jerschow, a specialist in allergy and immunology at Albert Einstein College of Medicine and Montefiore Medical Center. She studies adverse reactions to medications. Ketorolac and ibuprofen belong to a class of medications called NSAIDs — nonsteroidal anti-inflammatory drugs. These medications are a common cause of allergic and other types of hypersensitivity reactions, second only to antibiotics. There are different types of hypersensitivity reactions, and knowing which type the patient had was important for treatment and prognosis. If a patient has a true medication allergy, then the reaction will be triggered by that medication and no other — there is something about the chemical structure of that drug that tricks the immune system into acting as if the drug were an infection. It's uncommon, however, to develop a true allergy to NSAIDs. Far more common are nonallergic reactions to aspirin and other NSAIDs that are caused not by the chemical structure of the medication but by how it does the job it is meant to do. NSAIDs interrupt the chemical chain that leads to an inflammatory response; that's how they relieve pain. But for reasons that are not well understood, in some people this chemical chain changes, and those alterations cause the opposite to happen. Instead of reducing swelling, NSAIDs in these patients increase it, causing tissues, particularly in the respiratory tract, to become inflamed and swollen. Many of these reactions look just like an allergic response. Patients may get hives, itchy eyes and a runny nose. Or swelling of the eyes, lips or throat. Unlike true allergic reactions, which are usually limited to a single medication, this reaction can occur when any of the painkillers that affect this part of the chemical chain are taken. For most of these reactions — both allergic and nonallergic — simply avoiding the medication, or the class of medication, is sufficient to prevent the consequences. But there is one type of nonallergic reaction to NSAIDs for which avoiding the drug is not enough. In what's called aspirin-exacerbated respiratory disease (AERD), the hypersensitivity reaction these medications trigger is an essential clue to an underlying progressive disease process that can and should be treated. AERD is characterized by the confluence of three disorders that together are known as Samter's triad: First, patients have a hypersensitivity to aspirin and other NSAIDs; second, they have asthma that is often poorly controlled, even on the usual medications; third, they have nasal polyps. Although the disorder was first described early in the 20th century, it is still the focus of much research and is only beginning to be understood. A Clue in the NoseJerschow suspected that the patient had AERD. Her recent hospitalization proved that she had a severe hypersensitivity reaction to NSAIDs. And she had poorly controlled asthma too. But did she have nasal polyps? Not that she knew of, the patient said. How was her sense of smell? the doctor asked. Over the past year or so, the patient reported, she'd lost her sense of smell — and much of her sense of taste. She was a good cook, but without the ability to smell or taste, it was hard to tell how a dish turned out. Jerschow wasn't surprised; loss of smell is seen in over 95 percent of patients with AERD. Jerschow put the nasal speculum into the woman's nose. Were there polyps? Jerschow wasn't sure. She sent the patient to an ear, nose and throat specialist who scoped her upper airways. She did indeed have polyps. She had AERD. It's a common disorder; up to 15 percent of those with severe asthma have AERD. The abnormality in the inflammatory pathway these patients develop somehow drives the entire process. It causes their hard-to-control asthma. It propels the development of nasal polyps. And it underlies these patients' upside-down response to NSAIDs. Finding a Helpful TreatmentStrangely, the treatment for AERD is aspirin, the oldest member of the NSAID family. It's not clear why, but a daily dose of aspirin, titrated as high as is tolerated without triggering symptoms, can both improve asthma control and slow down the regrowth of nasal polyps. The patient tried taking aspirin for several months, but nothing improved. In the five years since she was given this diagnosis, she has tried several medications. Some helped her sense of smell and taste, but none allowed her to start running — much less racing. A couple of months ago, the patient started a new medicine called Dupilumab. It's a drug that targets a key step in the abnormal pathway to inflammation and swelling. She already feels much better. She'll know she's really back to her old self once she's tough enough for her next Tough Mudder. |
| High-Dose Vitamin D Shows No Benefit in the Critically Ill - Medscape Posted: 12 Dec 2019 09:50 AM PST 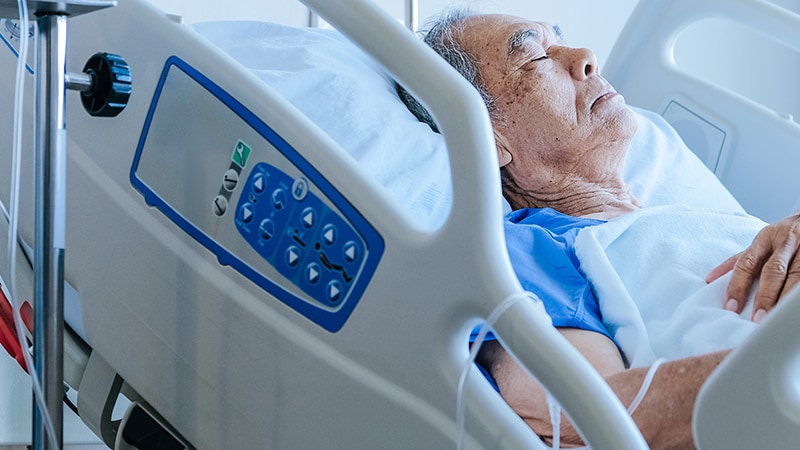 Critically ill patients who are vitamin D deficient do not seem to gain any benefit from the administration of high-dose vitamin D in the intensive care unit, results from a phase 3 randomized trial indicate. "Early administration of high-dose enteral vitamin D3 did not provide an advantage over placebo with respect to 90-day mortality or other nonfatal outcomes among critically ill, vitamin D–deficient patients," concludes the National Heart, Lung, and Blood Institute (NHLBI) Prevention and Early Treatment of Acute Lung Injury (PETAL) Network, which conducted the study. The findings were published online December 11 in the New England Journal of Medicine. With studies linking vitamin D deficiency to morbidity as well as mortality in critical illness, supplementation with high doses of vitamin D has gained interest as potentially having some benefit, and smaller studies have suggested some reductions in mortality with vitamin D administration among critically ill patients. Over 1000 Critically Ill Patients RandomizedTo evaluate the issue in a large population, the PETAL Network conducted the Vitamin D to Improve Outcomes by Leveraging Early Treatment (VIOLET) trial, enrolling critically ill patients who were at a high risk of death at 44 US hospitals. Of those patients, 1078 had vitamin D deficiency confirmed on subsequent liquid chromatography–tandem mass spectrometry testing and were included in the primary analysis. Patients were randomized within 12 hours of the decision to admit to the intensive care unit (ICU) from the emergency department, hospital ward, operating room, or outside facility to receive a single enteral dose of 540,000 IU of vitamin D3 or matched placebo; 1059 patients received the assigned treatment. At day 3 following administration, the mean level of 25-hydroxyvitamin D in the supplement group increased to 46.9 ± 23.2 ng/mL and the level in the placebo group was 11.4 ± 5.6 ng/mL. The 90-day mortality rates in the primary analysis population were 23.5% in the vitamin D group (125 of 531 patients died) and 20.6% in the placebo group (109 of 528 patients died); the difference was not significant (P = .26). The data and safety monitoring board recommended the trial be stopped at the first interim analysis on the basis of a probability of less than 2% that vitamin D treatment would be found superior to placebo in the full trial enrolment population. The two groups showed no significant differences in any other secondary clinical, physiological, or safety endpoints, such as length of hospital stay, ventilator-free days, or change in the five-level EuroQol 5 dimensions (EQ-5D-5L) score. Importantly, predefined subgroups of interest, including those with more severe vitamin D deficiency and specific acute risk factors for death, also gained no benefit from vitamin D supplementation. Early Testing for, or Treatment of, Vitamin D Deficiency Not SupportedConversely — and unexpectedly — a higher mortality rate was in fact observed in the vitamin D supplemented patients whose illnesses had infectious causes, and those who had acute respiratory distress syndrome prior to randomization. Several factors could explain those findings, the authors note. "This observation may reflect differences between the use of vitamin D for prevention in previous studies and the use of vitamin D as treatment during acute illness in the present trial, but it also may be the result of chance," they say. In the previous phase 2 VITdAL-ICU study published in 2014, 475 vitamin D–deficient, critically ill patients had lower mortality with supplementation; however, the difference was not significant compared with placebo at 28 days (21.9% vs 28.6%; P = .14) and at 6 months (35.0% vs 42.9%; P = .09). The authors of the current study note that there are important differences between their trial and VITdAL-ICU, such as the fact that patients in the new trial were enrolled earlier in their critical illness and had typical medical ICU illnesses, such as pneumonia, sepsis, shock, or respiratory failure, whereas most patients in the phase 2 trial were surgical or neurologic ICU patients. And in the earlier trial 99% of patients were white, whereas the new study was racially diverse and representative of the US population. "Given known differences in vitamin D metabolism and response genes according to race and ethnic group, such differences may affect the results," the authors write. They conclude, however, that "the results of the present trial do not support early testing for, or treatment of, vitamin D deficiency in critically ill patients." N Engl J Med. Published online December 11, 2019. Abstract For more diabetes and endocrinology news, follow us on Twitter and Facebook. |
| You are subscribed to email updates from "respiratory drugs,respiratory failure causes,respiratory failure treatment" - Google News. To stop receiving these emails, you may unsubscribe now. | Email delivery powered by Google |
| Google, 1600 Amphitheatre Parkway, Mountain View, CA 94043, United States | |
Comments
Post a Comment