Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: a proof of concept and mechanism study | Molecular Psychiatry - Nature.com
Abstract
The immune-inflammatory response during the acute phase of COVID-19, as assessed using peak body temperature (PBT) and peripheral oxygen saturation (SpO2), predicts the severity of chronic fatigue, depression and anxiety symptoms 3–4 months later. The present study was performed to examine the effects of SpO2 and PBT during acute infection on immune, oxidative and nitrosative stress (IO&NS) pathways and neuropsychiatric symptoms of Long COVID. This study assayed SpO2 and PBT during acute COVID-19, and C-reactive protein (CRP), malondialdehyde (MDA), protein carbonyls (PCs), myeloperoxidase (MPO), nitric oxide (NO), zinc, and glutathione peroxidase (Gpx) in 120 Long COVID individuals and 36 controls. Cluster analysis showed that 31.7% of the Long COVID patients had severe abnormalities in SpO2, body temperature, increased oxidative toxicity (OSTOX) and lowered antioxidant defenses (ANTIOX), and increased total Hamilton Depression (HAMD) and Anxiety (HAMA) and Fibromylagia-Fatigue (FF) scores. Around 60% of the variance in the neuropsychiatric symptoms of Long COVID (a factor extracted from HAMD, HAMA and FF scores) was explained by OSTOX/ANTIOX ratio, PBT and SpO2. Increased PBT predicted increased CRP and lowered ANTIOX and zinc levels, while lowered SpO2 predicted lowered Gpx and increased NO production. Lowered SpO2 strongly predicts OSTOX/ANTIOX during Long COVID. In conclusion, the impact of acute COVID-19 on the symptoms of Long COVID is partly mediated by OSTOX/ANTIOX, especially lowered Gpx and zinc, increased MPO and NO production and lipid peroxidation-associated aldehyde formation. The results suggest that post-viral somatic and mental symptoms have a neuroimmune and neuro-oxidative origin.
Introduction
Patients who have recovered from SARS-CoV-2 infection and have been discharged from the hospital may experience symptoms for much longer than expected, a condition known as "Long COVID." [1,2,3,4,5,6]. Numerous other terms for post-COVID symptoms have been suggested, including post-acute COVID, protracted COVID, chronic post-COVID, and Long Haul COVID [7, 8]. The most often used definition is: symptoms persisting for more than 3 months from the onset of acute COVID-19 [9, 10]. Extended symptoms have been classified as probably infection-related (up to 4–5 weeks), acute post-COVID symptoms (weeks 5–12), prolonged post-COVID symptoms (weeks 12–24), and chronic post-COVID symptoms (more than 24 weeks) [7].
Following the acute phase of COVID-19, the presence of more than one symptom is quite prevalent, occurring in 74% [11, 12] to 87.4% of all infected patients [12]. Initially, patient concerns were dismissed as mental health problems, including worry or stress [13], but later it became clear that people who suffer from long-term COVID have a wide range of physical and mental symptoms [14,15,16]. Among the various symptoms associated with Long COVID, the most frequently reported symptoms are fatigue and dyspnoea [9, 11, 17, 18], post-traumatic stress symptoms [19, 20], concentration and memory problems [21, 22], and anxiety and depression [4, 23]. Within 6 months after the first COVID-19 symptom, almost a third of COVID-19 survivors had a neuropsychiatric diagnosis such as insomnia, anxiety and depression [24].
Recently, we reported that not only the acute infectious phase [25] but also Long COVID [26] is characterized by concurrent elevations in depression (depressed mood, feelings of guilt, suicidal ideation, loss of interest), anxiety (anxious mood, tension, fears, anxiety behavior at interview), chronic fatigue and somatic symptoms including autonomic and gastrointestinal (GIS) symptoms, malaise and muscle pain. In addition, in both the acute phase and Long COVID, a single latent vector could be derived from these somatic and affective symptoms, demonstrating that these symptom profiles are the expression of a shared core, namely the acute COVID-19 and Long COVID neuropsychiatric symptoms [25, 26].
We reported that in acute COVID-19, the neuropsychiatric symptoms were largely predicted by a latent factor derived from indicants of increased proinflammatory and immunoregulatory cytokines, chest computerized tomography scan abnormalities (CCTAs), including ground-glass opacities, crazy patterns and consolidation and lower oxygen saturation in peripheral blood (SpO2) [25]. Importantly, lowered SpO2 and increased peak body temperature (PBT) during the acute phase of illness largely predict the severity of the neuropsychiatric symptoms of Long COVID [26]. Both lowered SpO2 [26] and increased PBT [27] are indicants of the severity of the immune-inflammatory response of acute COVID-19, and both predict critical disease and mortality [27, 28]. Therefore, our findings indicate that the infectious-immune-inflammatory pathways during the acute phase of illness [29] largely predict the symptom core of Long COVID [26]. Nevertheless, there are no data on whether the biomarkers underpinning Long COVID are caused by the inflammatory responses during the acute phase.
Activated immune-inflammatory and oxidative and nitrosative stress (IO&NS) pathways may underpin the somatic and mental symptoms of Long COVID because chronic fatigue syndrome, major depression and generalized anxiety disorder (GAD) are characterized by activated IO&NS pathways. Thus, these disorders are accompanied by (a) signs of a mild inflammatory response as indicated by increased levels of C-reactive protein (CRP); (b) oxidative stress including increased activity of pro-oxidative enzymes including myeloperoxidase (MPO) and oxidative damage as indicated by elevated production of malondialdehyde (MDA), protein carbonyls (PCs) and advanced protein oxidation products (AOPP); (c) increased nitrosative stress as indicated by increased levels of nitric oxide (NO) metabolites and IgM directed to NO (nitroso) neoepitope adducts; and (d) indicants of lowered total antioxidant capacity (TAC) of plasma, glutathione peroxidase (Gpx) and zinc levels [30,31,32,33,34]. In chronic fatigue syndrome, depression and GAD, the neurotoxicity theories conceptualize that the effects of neuro-oxidative stress toxicity (OSTOX) coupled with lowered antioxidant (ANTIOX) defenses cause increased neurotoxicity leading to somatic and affective symptoms [30, 31]. This is supported by translational data indicating that blocking nitro-oxidative stress, inflammatory and neurotoxic pathways with selective antioxidative compouds may alleviate depressive-, anxiety-, and chronic fatigue-like behaviors [25, 26, 30, 35,36,37,38,39,40]. Moreover, in humans, treatment with selected antioxidants improves depression, anxiety and chronic fatigue symptoms [41,42,43,44].
Likewise, SARS-Cov-2 infection and acute COVID-19 are accompanied by an inflammatory response [29], increased MPO activity [45], indicants of oxidative damage [46,47,48] and increased NO production including increased levels of nitrotyrosine [49], and lowered antioxidant defenses (ANTIOX) as indicated by reduced TAC levels [45, 49], Gpx [50], and zinc [51]. Nevertheless, there are no data on whether the effects of the immune-inflammatory processes during acute infection (as indicated by lowered SpO2 and increased body temperature) on the neuropsychiatric symptoms are mediated by activated immune and O&NS (IO&NS) pathways.
Hence, the present study was performed to delineate whether the effects of SpO2 and PBT on the neuropsychiatric symptoms of Long COVID are mediated by IO&NS pathways, including CRP, the OSTOX/ANTIOX ratio and its indicators (MDA, AOPP, carbonyl proteins, NOx, nitrotyrosine, TAC, Gpx or zinc). In addition, the present work employs the precision medicine approach [51] to define a Long COVID model which links the acute inflammation of COVID-19 with IO&NS pathways and the neuropsychiatric symptoms 3–4 months later. Moreover, we intend to construct an endophenotype class [34] that integrates all those biomarkers with the neuropsychiatric symptoms of Long-COVID. These findings are necessary to comprehend the biology of Long COVID and post-viral symptoms in general and may aid in predicting who will develop chronic fatigue syndrome and affective symptoms as a result of COVID-19 and viral infections in general.
Subjects and methods
Subjects
We employed both a case-control research design (to explore differences between controls and Long COVID) and a retrospective cohort study design (to examine the effects of acute-phase biomarkers on Long COVID symptoms) in the current investigation. We recruited 120 patients in the last 3 months of 2021 who had at least two symptoms consistent with Long COVID and had previously been diagnosed and treated for acute COVID-19 infection. The patients were diagnosed using the WHO criteria of post-COVID (long COVID) [52], namely: (a) individuals having a history of proven SARS-CoV-2 infection, (b) symptoms persisted beyond the acute phase of illness or manifested during recovery from acute COVID-19 infection, (c) symptoms lasted at least 2 months and are present 3–4 months after the onset of COVID-19, and (d) patients suffer from at least two symptoms that impair daily functioning including fatigue, memory or concentration problems, shortness of breath, chest pain, persistent cough, difficulty speaking, muscle aches, loss of smell or taste, affective symptoms, cognitive impairment, or fever [52].
During the acute phase of the illness, all patients had been admitted to one of the official quarantine facilities in Al-Najaf city specialized in the treatment of acute COVID-19, including Al-Sader Medical City of Najaf, Al-Hakeem General Hospital, Al-Zahraa Teaching Hospital for Maternity and Pediatrics, Imam Sajjad Hospital, Hassan Halos Al-Hatmy Hospital for Transmitted Diseases, Middle Euphrates Center for Cancer, and Al-Najaf. Senior doctors and virologists made the diagnosis of SARS-CoV-2 infection and acute COVID-19 based on (a) presence of an acute respiratory syndrome and the disease's standard symptoms of fever, breathing difficulties, coughing, and loss of smell and taste; (b) positive reverse transcription real-time polymerase chain reaction findings (rRT-PCR); and (c) positive IgM directed to SARS-CoV-2. All patients showed, upon recovery from the acute phase, a negative rRT-PCR result.
We selected 36 controls from the same catchment area, who were either staff members or their family or friends. In addition, we included controls who tested negative for rRT-PCR and exhibited no clinical indications of acute infection, such as dry cough, sore throat, shortness of breath, lack of appetite, flu-like symptoms, fever, night sweats, or chills. Nevertheless, we selected the controls so that about one-third of them had distress or adjustment symptoms as a result of lockdowns and social isolation to account for their confounding effects, which are also seen in Long COVID patients. Thus, one-third of the controls showed Hamilton Depression Rating Scale (HAMD) [53] values between 7 and 12. A senior psychiatrist reviewed the lifetime history of neuropsychiatric disorders based on the clinical anamnesis and patient records. COVID patients and controls were excluded if they had a lifetime history of psychiatric axis-1 disorders, including major affective disorders such as major depression and bipolar disorder, dysthymia, GAD and panic disorder, schizo-affective disorder, schizophrenia, psycho-organic syndrome, and substance use disorders, except tobacco use disorder (TUD). Moreover, we excluded patients and controls who suffered from neurodegenerative and neuroinflammatory disorders, such as chronic fatigue syndrome [54], Parkinson's or Alzheimer's disease, multiple sclerosis, stroke, or systemic (auto)immune diseases such as diabetes mellitus, psoriasis, rheumatoid arthritis, inflammatory bowel disease and scleroderma, and liver and renal disease. In addition, pregnant and breastfeeding women were omitted from this study.
All controls and patients, or their parents/legal guardians, gave written informed permission before participation in the research. The research was approved by the University of Kufa's institutional ethics board (8241/2021) and the Najaf Health Directorate-Training and Human Development Center (Document No.18378/ 2021). The study was conducted following Iraqi and international ethical and privacy laws, including the World Medical Association's Declaration of Helsinki, The Belmont Report, the CIOMS Guideline, and the International Conference on Harmonization of Good Clinical Practice; our institutional review board adheres to the International Guidelines for Human Research Safety (ICH-GCP).
Clinical measurements
A senior psychiatrist used a semi-structured interview to collect socio-demographic and clinical data from controls and Long COVID patients 3–4 months after the acute infectious phase of COVID-19 (mean ± SD duration of illness was 14.68 ± 5.31 weeks). Three to four months after the onset of acute COVID-19, this senior psychiatrist assessed the following rating scales: (a) the 12-item Fibro-Fatigue (FF) scale to measure Chronic fatigue and fibromyalgia symptoms [55]; (b) the HAMD to assess the severity of depression [53]; and (c) the Hamilton Anxiety Rating Scale (HAMA) [56] to assess the severity of anxiety. However, all three scales encompass both mental (affective) and somatic symptoms; consequently, utilizing the total scores would produce equivocal findings about affective symptoms due to the influence of somatic symptoms on the total scores. As previously explained, we have calculated composites representing affective symptoms of the HAMA and HAMD and somatic symptoms of the HAMD, HAMA, and FF scores [25]. Thus, two HAMD subdomain scores were calculated: (a) depressive HAMD symptoms as the sum of sad mood + feelings of guilt + suicidal thoughts + loss of interest; and (b) somatic HAMD symptoms as the sum of somatic anxiety + GIS anxiety + genitourinary anxiety + hypochondriasis. Two HAMA subdomain scores were calculated: (a) anxiety HAMA symptoms, which were defined as anxious mood + tension + fears + anxiety behavior during the interview; and (b) somatic HAMA symptoms, defined as somatic sensory + cardiovascular + GIS + genitourinary + autonomic symptoms (respiratory symptoms were not included in the sum). We calculated a single somatic FF subdomain score as: muscular pain + muscle tension + fatigue + autonomous symptoms + GIS symptoms + headache + a flu-like malaise (thus excluding the cognitive and affective symptoms). We examined the psychometric properties of the first factor extracted from the somatic FF, HAMA, and HAMD scores as well as the affective HAMD and HAMA scores in order to determine if it is possible to construct a latent vector score that adequately reflects the overall neuropsychiatric symptoms (combining somatic and mental symptoms) [25, 26]. This is significant because, if a single valid vector can be identified (see below for criteria), it demonstrates that somatic and affective symptoms have a common core and, hence, a shared pathophysiology [25]. In addition, we constructed z-unit-based composite scores indicating autonomic symptoms, sleep problems, fatigue, GIS symptoms, and cognitive symptoms using all relevant HAMD, HAMA, and FF items (z transformed). TUD was diagnosed using DSM-5 criteria. We determined the body mass index (BMI) by dividing the body weight in kilograms by the squared height in meters.
Assays
Fasting blood samples were taken in the early morning between 7.30 and 9.00 a.m. after awakening and before having breakfast. Five milliliters of venous blood samples were drawn and transferred into clean plain tubes. Hemolyzed samples were rejected. After 10 min, the clotted blood samples were centrifuged for 5 min at 3000 rpm, and then serum was separated and transported into three new Eppendorf tubes until assay. Serum Gpx, NOx, MPO, MDA, AOPP, TAC, nitrotyrosine, and PCs were measured using ELISA kits supplied by Nanjing Pars Biochem Co., Ltd. (Nanjing, China). All kits were based on a sandwich technique and showed an inter-assay CV of <10%. Zinc in serum was measured spectrophotometrically using a ready for use kit supplied by Agappe Diagnostics Ltd., Cham, Switzerland. Consequently, we computed three composite scores: (a) oxidative stress toxicity (OSTOX) as the sum of z MDA + z AOPP + z carbonyl proteins + z MPO + z NOx + z nitrotyrosine; (b) ANTIOX as z TAC + z Gpx + z zinc; and (c) the OSTOX/ANTIOX ratio as z OSTOX – z ANTIOX. The laboratory operators were blinded to the clinical data.
A well-trained paramedical specialist measured SpO2 using an electronic oximeter supplied by Shenzhen Jumper Medical Equipment Co. Ltd., and a digital thermometer was used to assess body temperature (sublingual until the beep). We collected these indicators from patient records and analyzed the lowest SpO2 and PBT values recorded during the acute phase of illness. We created a new indicator based on these two evaluations that represents decreased SpO2 and increased PBT as the z transformation of the latter (z body temperature) - z SpO2 (dubbed the "TO2 index"). We recorded the immunizations received by each participant, namely AstraZeneca, Pfizer, or Sinopharm.
Statistics
Analysis of variance was performed to determine if there were variations in scale variables across groups, and analysis of contingency tables or Fisher's Exact Probability test were employed to determine connections between nominal variables. We used Pearson's product-moment correlation coefficients to examine relationships between ONS biomarkers and SpO2, PBT, and clinical assessments. We employed a univariate general linear model (GLM) analysis to characterize the associations between classifications and biomarkers while accounting for confounding factors such as TUD, sex, age, BMI, and education. Equality of variance between study groups was tested using the Levene test. In addition, we obtained model-generated estimated marginal means (SE) values and used protected pairwise comparisons between group means using Fisher's least significant difference. Multiple comparisons were examined using a p correction for false discovery rate (FDR) [57]. Multiple regression analysis was used to determine the important IO&NS biomarkers or cofounders that predict somatic and affective measures. We used an automated stepwise technique with a 0.05 p value to enter and p = 0.06 to remove. We calculated the standardized beta coefficients for each significant explanatory variable using t statistics with exact p value, as well as the model F statistics and total variance explained (R2). In addition, we examined multicollinearity using the variance inflation factor and tolerance. We checked for heteroskedasticity using the White and modified Breusch-Pagan tests, and where necessary, we generated parameter estimates with robust errors. Associations between biomarkers and clinical data were assessed in an unrestricted study group consisting of patients and controls combined (the primary analyses). We also assessed the associations in the restricted group of Long COVID patients only (the secondary analyses). The tests were two-tailed, and statistical significance was defined as a p value of 0.05. Using an effect size of 0.23, a p value of 0.05, a power of 0.8, and three groups with up to five variables in an analysis of variance, the sample size should be around 151 participants (estimated using GPower 3.1). As a result, we enrolled 156 individuals, 36 controls and 120 Long COVID participants.
The precision medicine approach
By integrating biomarker and clinical data, we hoped to create endophenotype classes for Long COVID patients (using cluster analysis) and novel pathway phenotypes (using factor analysis). We conducted two-step cluster analyses on categorical (e.g., diagnosis) and scale variables (e.g., all biomarkers) to define new meaningful clusters of Long COVID patients. When the silhouette measure of cohesiveness and separation was more than 0.4, the cluster solution was deemed satisfactory. We conducted exploratory factor analysis (unweighted least-squares extraction, 25 iterations for convergence) and assessed factorability using the Kaiser-Meier-Olkin (KMO) sample adequacy metric (adequate when >0.7). In addition, when all loadings on the first factor were >0.6, the variance explained by the first factor was >50.0%, and the Cronbach alpha on the variables was >0.7; the first factor was considered a validated latent construct underlying the variables. Canonical correlation analysis (CCA) was used to investigate the associations between two sets of variables, with symptoms 3–4 months following the acute phase serving as the dependent variable set and both biomarkers of the acute and Long COVID phases as the explanatory variable set. We calculated the variance explained by the canonical variables in both sets, and we accept the canonical sets as an overall measure of the underlying construct when the explained variance is >0.50 and all canonical loadings are >0.5. Finally, we also compute the variance explained in the dependent clinical set by the biomarker set. All statistical analyses were conducted using IBM SPSS Windows, version 28.
Smart PLS analysis [58] was utilized to investigate the causal links between the lowest SpO2 and PBT in the acute phase and the neuropsychiatric symptoms in Long COVID, whereby the effects of SpO2 and temperature are mediated by IO&NS biomarkers. All input variables were entered as single indicators, while the output variable was a latent vector extracted from somatic FF, HAMD and HAMA, and affective HAMD and HAMA scores. Complete PLS analysis was performed only when the inner and outer models met predefined quality criteria, namely: (a) the output LV has high composite reliability >0.7, Cronbach's alpha >0.7, and rho A > 0.8 with an average variance extracted (AVE) > 0.5, (b) all LV loadings are >0.6 at p < 0.001, (c) the model fit is <0.08 in terms of standardized root mean squared residual (SRMR), (d) confirmatory tetrad analysis shows that the LV was not incorrectly specified as a reflective model, (e) blindfolding shows that the construct's cross-validated redundancy is adequate, and (f) the model's prediction ability as evaluated using PLSPredict is adequate. If the model quality data are adequate, we perform a complete PLS pathway analysis using 5000 bootstrap samples and compute the path coefficients with exact p value, as well as specific and total indirect (mediated) effects and total effects.
Results
Construction of an endophenotype class based on all biomarkers
To discover new endophenotype classes of Long COVID patients, we used a two-step cluster analysis with the diagnosis (Long COVID versus controls as a category) and the acute COVID-19 biomarkers SpO2 and body temperature and Long COVID biomarkers OSTOX, ANTIOX, OSTOX/ANTIOX and CRP as continuous variables. Nevertheless, the solution without CRP was much better, and, therefore, CRP was deleted from the model. We found a three-group model with an adequate measure of cohesion and separation of 0.57, namely controls (n = 36) and two patient clusters comprising 67 (cluster 1) and 51 (cluster 2) patients, respectively. Table 1 shows the socio-demographic and biomarker data of the three clusters. Cluster 2 patients showed lower SpO2, zinc, Gpx and ANTIOX levels and higher body temperature, OSTOX, OSTOX/ANTIOX, NOx, MPO, MDA and protein carbonyl levels as compared with cluster 1 patients. As such, the Long COVID group is divided into two clusters, one (cluster 2) with highly activated O&NS pathways (LC + O&NS) and one with less severe aberrations (LC). The LC group was differentiated from controls by increased body temperature and CRP and lowered SpO2, Gpx and ANTIOX values. There were significant differences between both Long COVID classes in Gpx (F = 12.79, df = 1/116, p < 0.001), NO (F = 37.98, df = 1/116, p < 0.001), PCs (F = 8.89, df = 1/116, p = 0.003), MPO (F = 23.72, df = 1/116, p < 0.001), MDA (F = 9.88, df = 1/116, p = 0.002), zinc (F = 5.79, df = 1/116, p = 0.018), OSTOX (F = 106.16, df = 1/116, p < 0.001), ANTIOX (F = 16.96, df = 1/116, p < 0.001), OSTOX/ANTIOX (F = 113.10, df = 1/116, p < 0.001), and CRP (F = 7.62, df = 1/116, p = 0.007), whilst AOPP (F = 2.74, df = 1/116, p = 0.101), TAC (F = 1.10, df = 1/116, p = 0.296), and nitrotyosine (F = 0.73, df = 1/116, p = 0.396) were not significant. P correction for FDR did not change any of these significant intergroup differences. Moreover, the ANTIOX index was significantly lower in both patient groups as compared with controls, and also in the total Long COVID group as compared with controls (F = 17.46, df = 1/154, p < 0.001).
Table 1 demonstrates the socio-demographic data in Long COVID patients divided into LC and LC + O&NS. No significant differences among these study groups were detected in BMI, residency, employment, education, vaccination status, and TUD. Age was somewhat higher in the LC + O&NS group, and there were more males in the LC + O&NS group than in the LC group. The disease duration was somewhat higher in LC + O&NS as compared with LC.
The same table also shows the medications patients were treated with during acute COVID-19 infection (enoxaparin, dexamethasone, ceftriaxone, azithromycin, bromhexin, levoflaxacin) and Long COVID (antidepressants, namely sertraline (n = 16), fluoxetine (n = 7) and imipramine (n = 6), propanolol, chlordiazepoxide). We have examined the effects of these treatments on the rating scale scores and all biomarkers used in this study, but no significant effect of these treatments could be found (even without p correction for FDR), whilst the intergroup differences in rating scales and biomarkers remained significant (results of multivariate GLM).
Associations between LC clusters and symptoms of Long COVID
Table 2 shows the results of univariate GLM analysis examining the associations between diagnosis into controls, LC and LC + IO&NS and all clinical scores while controlling for the effects of age, sex, education years, and TUD (entered as covariates). We found that the total FF, HAMD and HAMA, somatic FF, HAMD and HAMA scores, depression HAMD and anxiety HAMA, as well as the autonomic and GIS symptoms, were significantly different between the three classes and increased from controls → LC → LC + O&NS. Sleep disorders, chronic fatigue and cognitive disorders were significantly higher in Long COVID than in controls.
Table 3 shows the intercorrelation matrix of the OSTOX/ANTIOX ratio and SpO2, PBT, and clinical ratings in the total study group and Long COVID patients separately. The OSTOX /ANTIOX ratio was significantly associated with all symptom scores. There were no significant associations between CRP and the OSTOX/ANTIOX ratio. Figure 1 shows the partial regression of the neuropsychiatric symptoms score on OSTOX/ANTIOX in the total sample after adjusting for age, sex, BMI, education and TUD. All significant correlations shown in this table remained significant after p correction for FDR.
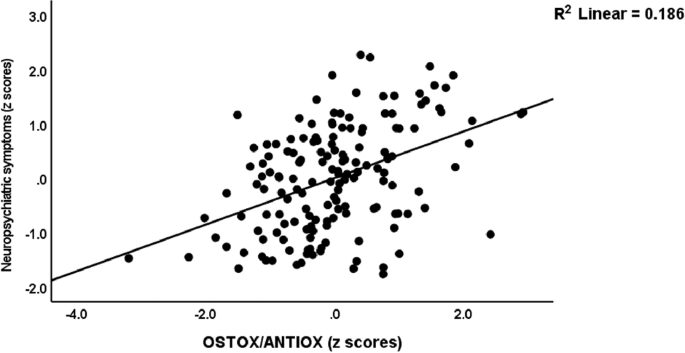
Partial regression of the neuropsychiatric symptoms score on the oxidative stress toxicity/antioxidant (OSTOX/ANTIOX) ratio in patients with Long COVID and normal controls combined.
Prediction of the symptom scores by IO&NS biomarkers
Table 4 shows the results of multiple regression analyses with somatic and affective measurements as dependent variables and O&NS biomarkers and CRP as explanatory variables while allowing for the effects of age, sex, BMI, TUD, education and vaccination types. Regression #1 shows that 25.0% of the variance in the depression HAMD score could be explained by the regression on Gpx and education (inversely associated) and MDA, CRP and carbonyl proteins (positively associated). We found that 23.0% of the variance in the somatic HAMD score (regression #2) was explained by the cumulative effects of lowered Gpx and zinc, increased NO and CRP and vaccination with AstraZeneca or Pfizer. Regression #3 shows that 13.1% of the variance in anxiety HAMA is explained by CRP, female sex and AstraZeneca vaccination. In Regression #4, 26.7% of the variance in somatic HAMA was explained by the regression on CRP and NO (positively) and Gpx (inversely), female sex and vaccination with AstraZeneca or Pfizer. Regression #5 shows that 28.0% of the variance in somatic FF was explained by NO, MDA, CRP (positively) and Gpx (inversely). Up to 30.2% of the variance in the severity of the neuropsychiatric symptom score was explained by NO, MDA, CRP (positively), Gpx (inversely), female sex, and vaccination with AstraZeneca or Pfizer.
Prediction of symptom scores by IO&NS biomarkers, SpO2 and body temperature
We have rerun the analyses shown in Table 4 and include the OSTOX, ANTIOX, OSTOX/ANTOX ratio, SpO2 and body temperature and the results are presented in Table 5. SpO2 was inversely associated with all 6 somatic and affective scores (regressions #1–#6), body temperature was positively associated with all scores, except anxiety HAMA, while IO&NS biomarkers had significant effects on all scores above and beyond the effects of SpO2 and body temperature (all except anxiety HAMA). OSTOX was associated with depression HAMD, NO with somatic HAMD HAMA and FF, MDA predicted somatic FF and OSTOX/ANTIOX predicted the neuropsychiatric symptoms. The effects of CRP disappeared after considering the effects of the other explanatory variables.
In this Table, we also examine whether the OSTOX/ANTOX ratio and CRP are predicted by SpO2 and body temperature while allowing for the effects of confounders. Regressions #7 and 8 display that the OSTOX/ANTIOX ratio was predicted by SpO2 and CRP by body temperature and male sex. Figure 2 shows the partial regression of the OSTOX/ANTIOX ratio on SpO2 in the patient group in Long COVID and control participants combined. Figure 3 shows the partial regression of serum CRP on PBT during the acute phase of COVID-19 (performed in Long COVID and control participants combined). In addition, we have calculated the regressions of the same clinical rating scales on the biomarkers for participants with Long COVID only. Table 1 of the Electronic Supplementary File (ESF, Table 1) provides a summary of the results. The regression on SpO2, OSTOX (NO), and ANTIOX (zinc, Gpx) markers, vaccination with AstraZeneca and Pfizer, and female sex explained a significant proportion of the variation in these clinical rating scales in subjects with Long COVID.
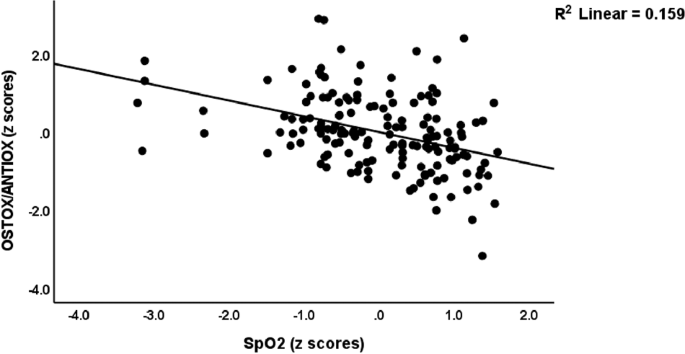
Partial regression of the oxidative stress toxicity/antioxidant (OSTOX/ANTIOX) ratio in Long COVID patients on peripheral blood oxygen saturation (SpO2) during the acute phase of COVID-19. This regression is performed in Long COVID and control participants.
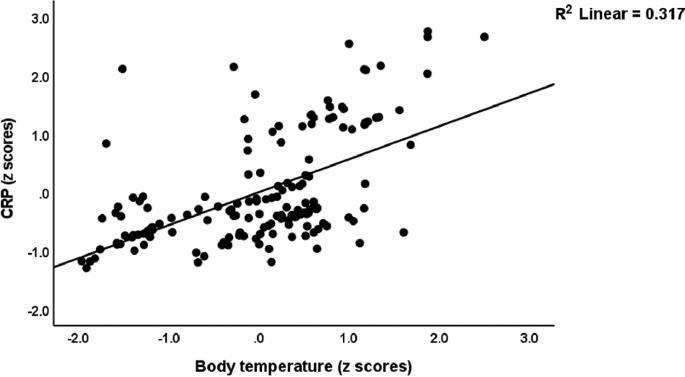
Partial regression of serum C-reactive protein (CRP) in Long COVID patients on peak body temperature during the acute phase of COVID-19. This regression is performed in Long COVID and control participants.
Associations between somatic and affective scores and all biomarkers combined
To examine the association between the combined effects of SpO2, body temperature and the biomarkers of Long COVID, we performed two types of analyses: (a) Pearson's correlation analyses between the somatic and affective scores and a new composite score computed as z body temperature - SpO2 + z OSTOX – z ANTIOX (dubbed the BTO2ONS index); and (b) canonical correlation analyses with the clinical scores as dependent variables and SpO2, body temperature and a Long COVID biomarker composite score (z OSTOX + z CRP – z ANTIOX, dubbed the ONSCRP index) as explanatory variables.
Table 3 shows that the BTO2ONS index was significantly correlated with CRP and with all clinical scores in the total study group and the restricted study group of Long COVID patients (except for cognitive disorders). Figure 4 shows the partial regression of the neuropsychiatric symptom score on the BTO2ONS composite score after controlling for age, sex, education and TUD in Long COVID patients. ESF Fig. 1 and Fig. 2 show the regressions of the somatic HAMA and FF scores, respectively, on the BTO2ONS index in Long COVID patients.
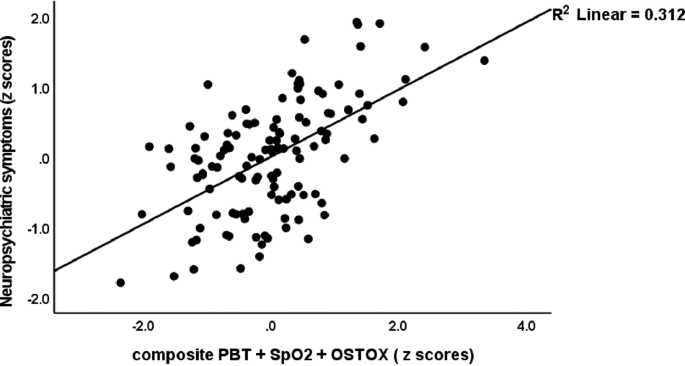
Partial regression of the neuropsychiatric symptoms score in Long COVID patients on a composite score based on increased oxidative stress toxicity and reduced antioxidant defences, peak body temperature and peripheral oxygen saturation (PBT + SpO2 + OSTOX). This regression is performed in Long COVID participants only.
Table 6 shows the results of three canonical correlations with different sets of neuropsychiatric symptom scores as dependent variables. All CCAs showed that one canonical function was significant, while the other variates had no significant information about the variables. CCA #1 shows that the first canonical correlation between the biomarkers and total symptom scores was r = 0.752. The first canonical variate based on SpO2, body temperature and the ONSCRP index explained 64.6% of the variance, indicating that this component is an overall measure of the adverse outcome pathways, and the first canonical variate based on the rating scale scores explained 89.4% of the variance indicating it is an overall measure of severity of the neuropsychiatric symptoms of Long COVID. Moreover, the amount of variance explained by the pathways in the total neuropsychiatric symptoms was 50.6%. The same biomarker set explained 41.6% of the variance in a canonical variate extracted from somatic FF, HAMA and HAMD scores and anxiety HAMA and depression HAMD scores, and 39.1% in a canonical component based on autonomic and GIS symptoms, insomnia, and chronic fatigue, while the canonical loading of cognitive disorder was lower (0.421).
PLS and construction of a new endophenotypic class and a pathway phenotype
Figure 5 shows a PLS model that examined whether the effects of SpO2 and body temperature during the acute phase of the neuropsychiatric symptoms of Long COVID (a latent vector extracted from the 5 rating scale subdomains) are mediated by IO&NS biomarkers. Therefore, we entered all ONS biomarkers and CRP as single indicators that could be predicted by SpO2 or PBT, and whereby the ONS biomarkers, the overarching OSTOX/ANTIOX ratio and CRP were allowed to predict the neuropsychiatric symptoms, which was entered as a latent vector extracted from somatic FF, HAMD and HAMA scores and depression HAMD and anxiety HAMA scores. With an SRMR of 0.027, the model quality was adequate, and we found adequate values for construct reliability validity of the neuropsychiatric symptoms (AVE = 0.731; composite reliability = 0.931, Cronbach alpha = 0.907, rho_A = 0.920, all loadings > 0.777). Blindfolding revealed appropriate construct cross-validated redundancy for the neuropsychiatric symptoms LV (0.424). Non-significant paths and non-significant indicators are deleted from the model. The following are the findings of the complete PLS analysis (bias-corrected and accelerated using 5000 bootstraps,) as shown in Fig. 5: a large part of the variance in the neuropsychiatric symptoms (60.3%) is explained by female sex, body temperature, SpO2 and OSTOX/ANTIOX ratio. The effects of SpO2 on the neuropsychiatric symptoms score are in part mediated by effects on Gpx (t = −2.21, p = 0.027) and NO (t = −2.05, p = 0.041) which contribute to the OSTOX/ANTIOX ratio. Body temperature (t = 4.02, p < 0.001), SpO2 (t = −9.14, p < 0.001), zinc (t = −2.74, p = 0.006), TAC (t = −2.90, p = 0.004), Gpx (t = −2.76, p = 0.006), NO (t = 2.75, p = 0.006), MDA (t = 2.85, p = 0.004), and MPO (t = 2.75, p = 0.006), PCs (t = 2.69, p = 0.007) have significant total effects on the output latent vector.
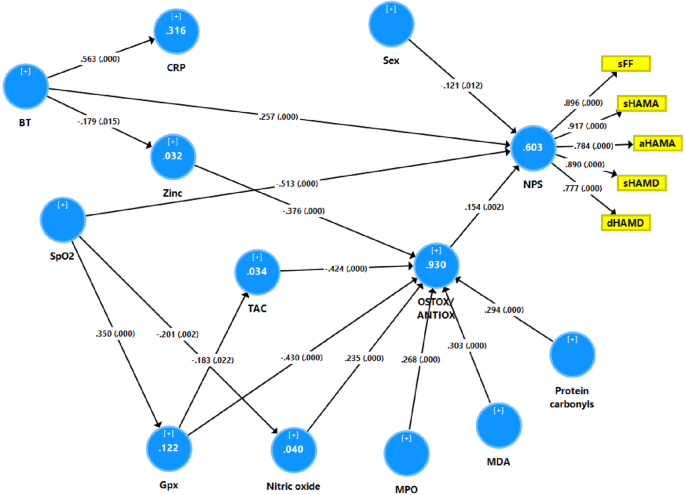
This model shows that the effects of reduced oxygen saturation (SpO2) and increased peak body temperature (PBT) during the acute phase of COVID-19 on the neuropsychiatric symptoms (NPS) of Long COVID are in part mediated by increased oxidative stress toxicity and lowered antioxidant defenses. The neuropsychiatric symptoms of Long COVID are entered as a latent vector extracted from subdomain scores on the Fibromylgia-Fatigue (FF), Hamilton Depression (HAMD) and Anxiety (HAMA) rating scales. sFF somatic FF symptoms, sHAMD and sHAMD somatic HAMD and HAMA scores, respectively, dHAMD depression HAMD scores, aHAMD anxiety HAMA scores, CRP C-reactive protein, Gpx glutathione peroxidase, TAC total antioxidant capacity, MPO m...
Comments
Post a Comment