“SARS-CoV-2 NSP14 induces potent inflammation via NF-κB pathways, finds study - News-Medical.Net” plus 2 more
“SARS-CoV-2 NSP14 induces potent inflammation via NF-κB pathways, finds study - News-Medical.Net” plus 2 more |
- SARS-CoV-2 NSP14 induces potent inflammation via NF-κB pathways, finds study - News-Medical.Net
- Antibody to SARS-CoV-2 inhibits complement hyperactivation - News-Medical.Net
- A Kidney Transplant Patient Who Died of COVID-19-associated Severe Acute Respiratory Distress Syndrome: A Case Report - DocWire News
| SARS-CoV-2 NSP14 induces potent inflammation via NF-κB pathways, finds study - News-Medical.Net Posted: 31 May 2021 09:43 AM PDT Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of the coronavirus disease 2019 (COVID-19) pandemic. SARS-CoV-2 has a single-stranded RNA genome encoding both structural and non-structural proteins. Of these, the latter is known to play a variety of roles in viral replication and the assembly of new viral particles, as well as in suppressing cellular immune responses. A new study reports the activity of one such protein, the non-structural protein 14 (NSP14), which activates the inflammatory mediator NF-κB to trigger intense inflammation. .jpg) A preprint version of the study is available on the bioRxiv* server, while the article undergoes peer review. BackgroundSARS-CoV-2 has been known to inhibit type I interferon (IFN) pathways while it triggers NF-κB-mediated pathways, thus triggering the production of inflammatory cytokines like interleukin (IL)-6 and IL-8. This is thought to underlie the intense systemic inflammation that is responsible for the extreme multi-system damage and respiratory failure seen in some cases of severe COVID-19. In order to understand how these inflammatory responses relate to NF-κB, the current study examined the regulatory activity of NSP14 in viral replication. This highly conserved protein has many functions, both in the replication of viral genomic and sub-genomic ribonucleic acid (RNA) and in modifying the latter, once synthesized. Functions of NSP14NSP14 is a proofreading exonuclease and thus limits mistakes during RNA synthesis by cutting out wrongly paired base pairs. It is also an RNA methyltransferase, transferring a methyl group to guanine at the N7 position. This activity, combined with NSP10/16-mediated 2'-O RNA methylation, is essential for the addition of a 5'-methyl cap to the new sgRNA strand to stabilize it against degradation by the ubiquitous host exonucleases, as well as disguise it against recognition by the host cell sensors of foreign RNA, such as RIG-1. Moreover, NSP14 enhances host ribosomal synthesis of viral proteins while reducing the amount of double-stranded RNA, which triggers the recognition of the pathogen-associated molecular pattern (PAMP) by the host cell receptors. Such recognition would otherwise activate antiviral responses. Finally, NSP14 encourages the recombination of viral RNAs to create new virus strains. Activity of NSP14 on NF-κB-mediated inflammationThe current study shows that NSP14 activates NF-κB-mediated inflammatory signaling pathways. Secondly, it increases the expression of IL-6 and IL-8. In COVID-19 patients, the levels of IL-6 and IL-8 were always higher than in controls. IL-8 is more strongly induced by NSP14 than IL-6 and was found to be expressed at higher levels in lung tissue from non-survivors of COVID-19. As with other viruses, viral replication may be enhanced by IL-8 expression. IL-8 expression is dependent on the formation of the NSP14-IMPDH2 (inosine-5'-monophosphate dehydrogenase 2) complex. IMPDH2 reduces cellular stress responses via its regulation of nucleotide synthesis within the cell. It is thought that the interaction of NSP14 with IMPDH2 may result in increased modification of host cellular RNAs, with both the exonuclease and methyltransferase activity of the former coming into play. In addition to promoting the nuclear translocation of the p65 molecule, NSP14 could also enhance its transcription and expression. Blockade of IMPDH2 prevents viral replicationIMPDH2 inhibition by the drugs ribavirin or mycophenolic acid resulted in a significant reduction of NF-κB activation by Nsp14, over a wide range of doses with these inhibitors. When IMPDH2 expression was blocked, IL-8 expression was likewise prevented. Conversely, higher levels of IMPDH2 did not affect NSP14-mediated NF-κB activation, irrespective of TNF-α. In vitro, these inhibitors also reduced the rate of infection of cells in culture by SARS-CoV-2, with both nucleocapsid protein and sgRNA levels showing a steep decline. Simultaneously, IL-8 levels also decreased. Earlier studies have confirmed that NSP14 suppresses host antiviral defenses by blocking type I IFN signaling and preventing the exit of IRF3 from the nucleus. What are the implications?The current study shows that the presence of NSP14 early in infection induces multiple cell pathways and promotes viral replication. The activation of NF-κB pathways leads to the release of other pro-inflammatory mediators to cause a cytokine storm, leading to acute respiratory distress syndrome (ARDS). Increased NF-κB expression and the resulting inflammation may benefit the virus by increasing host cell proliferation and survival or preventing cell death. NSP14 induces increased expression of IL-6 and IL-8, both potent immune cell recruiters. The resulting influx of neutrophils and macrophages may lead to further intensification of the hyperactive inflammatory response in the lung, causing severe injury. Thirdly, the role of the host protein IMPDH2 as a cofactor for NSP14 in the activation of NF-κB was confirmed. Not only was it found to interact with NSP14, as shown in earlier studies, but it promotes NF-κB activation. Thus, "Nsp14 may hijack IMPDH2 for NF-κB activation, contributing to abnormal inflammatory responses." This action may be the result of NSP14-mediated inhibition of the immunomodulatory role of IMPDH2. In all these cases, the use of the inhibitory molecules ribavirin and mycophenolic acid, both of which are already approved and in general clinical use, may be useful in treating COVID-19. The ongoing preclinical studies and clinical trials of these drugs against this illness may benefit from this increased understanding of the mechanism by which they act to disrupt the interactions between IMPDH2 and NSP14. *Important NoticebioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information. Journal reference: |
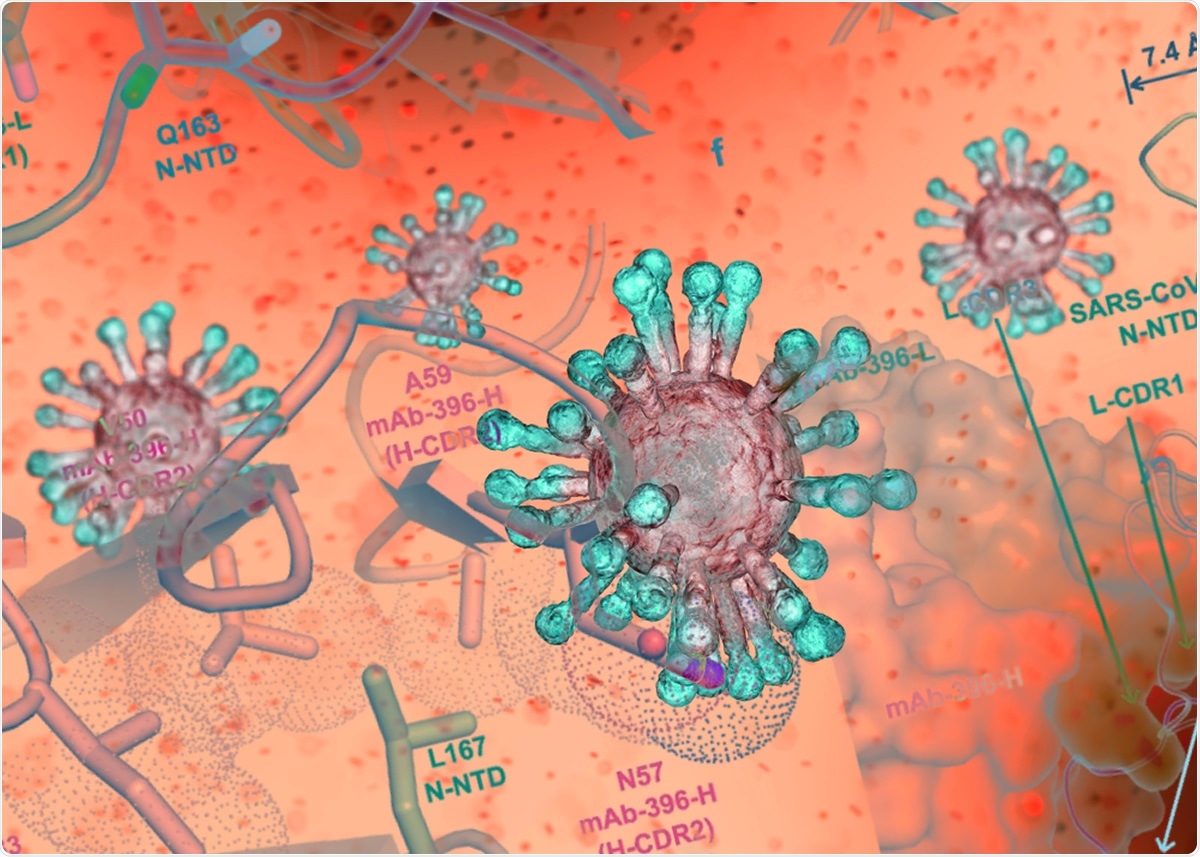
| Antibody to SARS-CoV-2 inhibits complement hyperactivation - News-Medical.Net Posted: 17 May 2021 12:00 AM PDT Researchers identified several antibodies to the nucleocapsid protein from a convalescent COVID-19 patient. Tests found some can inhibit complement hyperactivation and could potentially lead to new therapeutics. Approximately half of all patients with severe COVID-19 disease develop acute respiratory distress, which can be fatal. Some patients also have symptoms similar to acute respiratory distress syndrome (ARDS). Studies have suggested that a hyperactive complement system, a part of the immune system that increases the ability of antibodies to fight infections, predisposes patients to adverse COVID-19 outcomes. Hyperactivation of the complement system, which usually helps with immunity, can lead to tissue injury. The nucleocapsid protein of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is one of several proteins in the virus that perform numerous functions. Although several monoclonal antibodies against the SARS-CoV-2 spike protein have been developed and clinically tested, little is known about antibodies to the nucleocapsid protein. In a paper published recently in the journal Nature Communications, researchers report a human monoclonal antibody that targets the nucleocapsid protein. Antibodies to the nucleocapsid proteinThe team collected samples from six patients in China about 7–25 days after symptom onset. Through serological analysis, they found that the antibody levels against the nucleocapsid protein were higher than the antibody levels against the virus spike protein in most patients. The team found that the characteristics of the nucleocapsid antibodies recovered were different from those of spike antibodies during early recovery, suggesting sample collection time is critical in identifying the different immune responses to different viral proteins. These findings are consistent with those of other studies, some of which report antibodies to nucleocapsid protein developing earlier than spike antibodies and higher levels of nucleocapsid antibodies in patients requiring intensive hospital care. From the sample of one patient, who completely recovered within nine days of symptom onset, they found 32 monoclonal antibodies that reacted to the nucleocapsid protein, which included 20 antibodies from plasma cells and 12 from memory B cells. In contrast, they found only eight monoclonal antibodies to the spike protein. Of the antibodies that bind to the nucleocapsid protein, 13 bound to the N-terminal domain and one to the C-terminal domain. The team also determined the crystal structure for the complex with the nucleocapsid protein with one of the antibodies. The main interaction was found in the residues between 159 and 172 of SARS-CoV-2. The binding of the antibody causes several conformational changes in the N-terminal domain, leading to the binding pocket becoming bigger and partial unfolding of the basic palm region. Since some of the residues are conserved in all betacoronaviruses, this antibody may likely cross-react with other coronavirus nucleocapsid proteins. The authors confirmed this with binding studies, which showed that this antibody could also react with MERS-CoV. Inhibiting complement hyperactivationThe authors also developed a method to study levels of complement activation in the presence of the SARS-CoV-2 nucleocapsid protein. They found that the nucleocapsid protein induces hyperactivation of MASP-2 proteases, enzymes used in complement activation. Addition of a nucleocapsid antibody identified before reduced the rate of reaction of the complement hyperactivation. This inhibition was also seen with three other nucleocapsid protein antibodies identified. However, the exact interaction of the virus with protease is still unknown. There is growing evidence that severely ill COVID-19 patients benefit from the use of complement inhibitors, with several clinical trials underway. One of the nucleocapsid antibodies isolated has high binding to the protein and may potentially also inhibit complement hyperactivation. For the other antibodies identified, the team was unable to express the nucleocapsid protein regions they attach to separately as they belonged to disordered to flexible parts of the protein. The N- and C-terminal domains of the protein are involved in several key viral functions, and in the future, they plan to focus on antibodies binding to these regions. Journal reference: |
| Posted: 30 May 2021 11:00 PM PDT  This article was originally published here Intern Med. 2021 May 29. doi: 10.2169/internalmedicine.7089-21. Online ahead of print. ABSTRACT We herein report a 67-year-old kidney transplant patient who died of COVID-19. He was treated with hydroxychloroquine and azithromycin and received mechanical ventilation that temporarily improved his respiratory status. Despite our efforts, however, he later developed respiratory failure and died 43 days after the disease onset. The autopsy revealed prominent organization of alveoli and alveolar ducts, with a massive accumulation of macrophages in the lungs. A few SARS-CoV-2 antigen-positive cells were detected in the lung, suggesting delayed virus clearance owing to his long-term immunosuppressed state, leading to constant lung damage and ultimately respiratory failure. PMID:34053986 | DOI:10.2169/internalmedicine.7089-21 |
| You are subscribed to email updates from "what is respiratory distress,what is sars,what is sars virus" - Google News. To stop receiving these emails, you may unsubscribe now. | Email delivery powered by Google |
| Google, 1600 Amphitheatre Parkway, Mountain View, CA 94043, United States | |
Comments
Post a Comment