“Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): an exploratory, open-label, phase 2 randomised controlled trial - The Lancet” plus 3 more
- Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): an exploratory, open-label, phase 2 randomised controlled trial - The Lancet
- CRISPR screening identifies human host pathways that facilitate coronavirus infection - News-Medical.Net
- A teenager with fever, chest pain, and respiratory distress during the coronavirus disease 2019 pandemic: a lesson on anchoring bias - Wiley
- High-resolution structures of the SARS-CoV-2 2′-O-methyltransferase reveal strategies for structure-based inhibitor design - Science
| Posted: 28 Sep 2020 04:20 PM PDT [unable to retrieve full-text content]Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): an exploratory, open-label, phase 2 randomised controlled trial The Lancet |
| Posted: 27 Sep 2020 08:35 PM PDT The human coronavirus family consists of 7 known pathogens, for which there are no approved vaccines or specific therapeutic options. Although the seasonal human coronaviruses - OC43, HKU1, 229E, and NL63 – only cause mild respiratory infections, three highly pathogenic coronaviruses that emerged in the last two decades revealed the pandemic potential of this family of viruses. It is known that severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and Middle East respiratory syndrome coronavirus (MERS-CoV) can cause acute respiratory distress syndrome and even death. The fatality rates of these viruses ranged from 10 to 40%. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which caused the current COVID-19 pandemic, has a lower fatality rate but is far more transmissible than SARS-CoV-1 and MERS-CoV. So far, it has been responsible for nearly 33 million cases and 996,000 deaths worldwide. Given the severity of the impact of these viruses on human health, we must understand the mechanism behind the invasion of host cell machinery by SARS-CoV-2 and other coronaviruses during infection. Identifying host factors common to several coronaviruses could help develop therapies that can fight current and future pandemics caused by coronaviruses. CRISPR screens of infected cells reveal distinct viral entry factorsIn a recent preprint paper published on the preprint bioRxiv,* researchers from the University of California San Francisco, Gladstone Institutes, Chan Zuckerberg Biohub, and Synthego Corporation, San Francisco, discuss how they identified some host factors common to 3 coronaviruses with the help of the gene-editing tool, CRISPR. The team of researchers conducted parallel genome-wide CRISPR screens cells infected with SARS-CoV-2 and two seasonal common cold coronaviruses - OC43 and 229E. They were able to identify the distinct viral entry factors for all 3 viruses - ACE2 for SARS-CoV-2, glycosaminoglycans for OC43, and aminopeptidase N for 229E. Moreover, they also found that phosphatidylinositol phosphate biosynthesis and cholesterol homeostasis are critical host pathways that support infection by these three coronaviruses. TMEM106B, the lysosomal protein, was unique to SARS-CoV-2 infection. By pharmacological inhibition of cholesterol homeostasis and phosphatidylinositol phosphate biosynthesis, the authors were able to reduce the replication of all three coronaviruses. 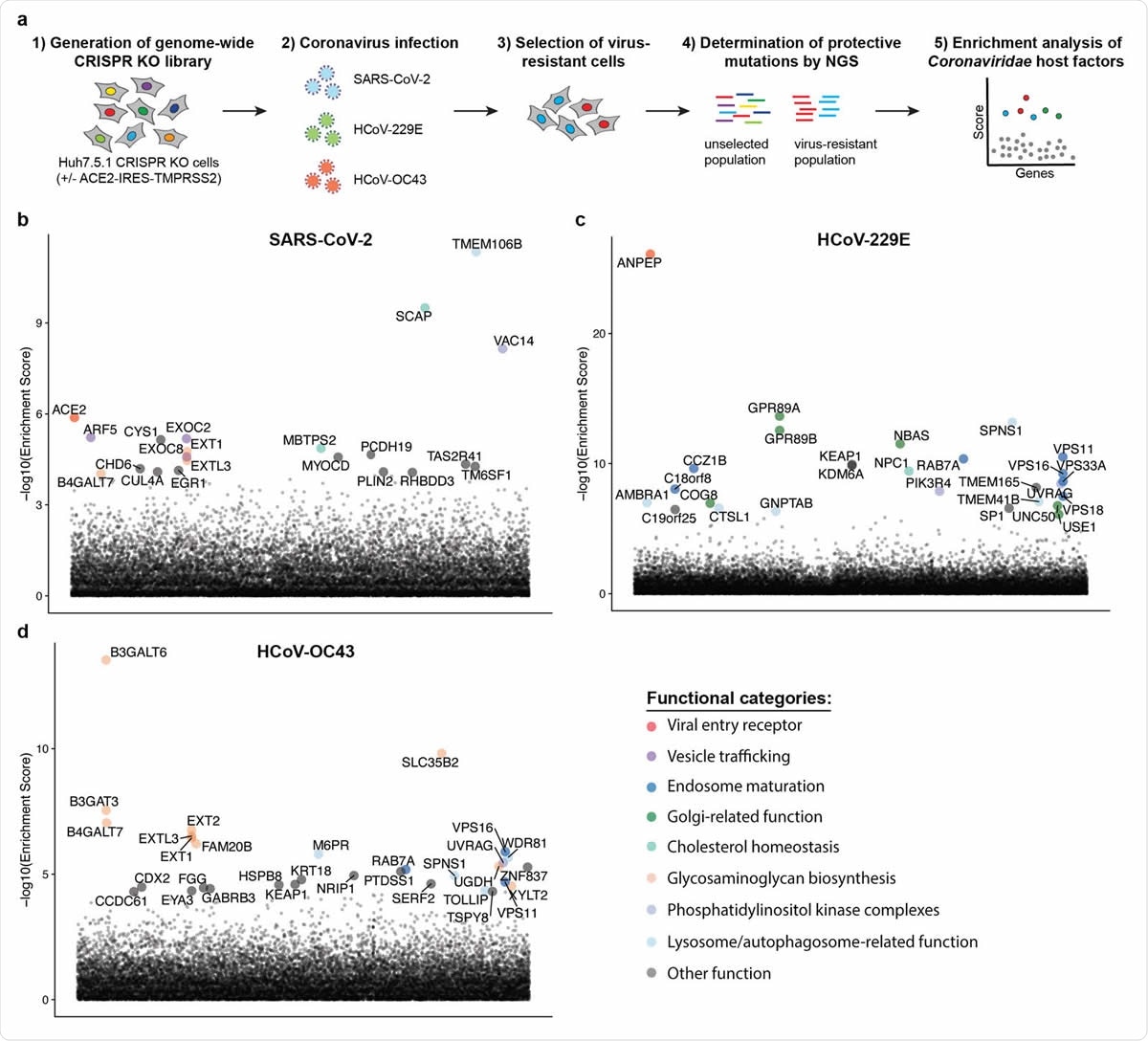 Genome-wide CRISPR KO screens in human cells identify host factors important for infection by for SARS-CoV-2, HCoV-229E and HCoV-OC43. (a) Schematic of CRISPR KO screens for the identification of coronavirus host factors. Huh7.5.1-Cas9 (with bicistronic ACE2-IRES-TMPRSS2 construct for SARS-CoV-2 and without for 229E and OC43 screen) were mutagenized using a genome-wide sgRNA library. Mutant cells were infected with each coronavirus separately and virus-resistant cells were harvested 10-14 days post infection (dpi). The abundance 347 of each sgRNA in the starting and selected population was determined by high-throughput sequencing and a gene enrichment analysis was performed. (b-d) Gene enrichment of CRISPR screens for (b) SARS-CoV-2, (c) 229E and (d) OC43 infection. Enrichment scores were determined by MaGECK analysis and genes were colored by biological function. The SARS-CoV-2 was performed once. The 229E and OC43 screens were performed twice and combined MaGECK scores are displayed. Data suggests unique entry factors but common host pathways for 3 coronavirusesThe results of the study highlight that while the three coronaviruses depend on unique entry factors, they have a common set of host pathways that help in infection. Genes linked to cholesterol homeostasis were highlighted in all screens and the network propagation. "Due to its involvement in multiple cellular processes including vesicular trafficking and autophagy, it remains to be determined whether coronaviruses hijack this pathway during entry or for the generation of double membrane vesicles required for the viral replication/transcription complexes." Consistent with the findings, 2 SARS-CoV-2 interactomes revealed viral proteins binding to SCAP, which was identified as a host factor that is critical for the replication of the SARS-CoV-2 virus. The researchers argue that given the role of SCAP in viral replication, viral proteins likely positively regulate SCAP activity and cholesterol levels. These findings provide key insights into the understanding of the life cycle of coronaviruses and important directions for the development of host-directed therapies to combat coronavirus infection. Previous studies have linked cellular cholesterol homeostasis to viral entry and membrane fusion in bunya- and hantavirus infections. This suggests a pro-viral function across different families of viruses. The team's findings were in agreement with this hypothesis. They were able to reduce infection with SARS-CoV-1 and CoV-2 spike-pseudotyped viruses by treatment with 25-hydroxycholesterol, which blocks SREBP processing and inhibits cholesterol synthesis. The results are also consistent with that of a recent drug repurposing screen that identified more than 100 compounds, including PIKfyve inhibitors, protease inhibitors, and cholesterol homeostasis modulators, that inhibited SARS-CoV-2 replication. The functional genomics data obtained by this study suggests that the observed effects of these compounds were potentially due to inhibition of crucial host factors. "Our results corroborate previously implicated host pathways, uncover new aspects of virus-host interaction, and identify targets for host-directed antiviral treatment." According to the team, the study offers critical insights into host pathways, usually hijacked by coronaviruses. They identified the phosphatidylinositol PIK3C3 kinase complex as a potential therapeutic target for SARS-CoV-2 based on the 229E and OC43 screens, highlighting the value of the parallel CRISPR screening for developing novel therapies against SARS-CoV-2 and other viruses of the Coronaviridae family. *Important NoticebioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information. Journal reference:
|
| Posted: 26 Sep 2020 02:33 PM PDT  1 CASE PRESENTATIONA previously healthy 17‐year‐old male presented to the emergency department (ED) with a 7‐day history of worsening symptoms, including left‐sided sore throat, chills, diarrhea, vomiting, diffuse body aches, right‐sided chest pain, and persistent fever. Throughout the past week, he had several outpatient and ED evaluations, including negative testing for strep pharyngitis, influenza, mononucleosis, and Epstein‐Barr virus. He also had 2 nasal swab specimens collected for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) virus testing. Both were polymerase chain reaction tests, and both were eventually negative. He had been discharged to home with advice on supportive care, including antipyretics and good oral hydration. On this presentation, he had a 97% pulse oximetry on room air, and he was febrile and tachycardic and had marked diastolic hypotension. He had erosions in the left posterior tonsil, an exudative oropharynx, diminished breath sounds bilaterally, and progressively worsening respiratory distress. Laboratory results demonstrated leukopenia as well as markedly elevated D‐dimer, C‐reactive protein, and procalcitonin. Chest radiograph showed bilateral nodular densities with ground‐glass, interpreted as multilobar pneumonia. He was fluid resuscitated, administered broad‐spectrum antibiotics, and given nasal cannula oxygen. He was admitted to an isolation floor for patients suspected to have coronavirus disease 2019 (COVID‐19) while his third SARS‐CoV‐2 virus polymerase chain reaction test was pending. A non‐occlusive thrombus of his left internal jugular vein was found on computed tomography imaging. His blood cultures revealed group G streptococcus, and he was initially treated with clindamycin, vancomycin, ceftriaxone, and ampicillin/sulbactam for 2 days. He was diagnosed with Lemierre's syndrome and group G strep sepsis. A later blood culture found Fusobacterium necrophorum; on this finding, his antibiotics were switched to meropenum. Throughout the length of his stay, the patient experienced severe chest pain, presumably from septic micro emboli. He received pain medication for his chest pain and had worsening hypoxemia and respiratory distress (accessory muscle use and increasing respiratory rates) during the first 3 days of his hospital stay. He had worsening edema on his chest radiograph without improvement with furosemide, was subsequently transferred to the pediatric ICU on hospital day 4, and was placed on bilevel positive airway pressure to maintain adequate oxygen levels. He had a peripherally inserted central catheter on hospital day 5. Despite antibiotics, he continued to have worsening symptoms, and an effusion developed on his chest radiograph. On hospital day 7, a chest tube was introduced into his right lung that drained punch‐colored sterile pleural fluid. Thereafter he was easily weaned off the oxygen support and was transitioned to room air. His D‐dimer increased further, and his laboratory results showed increasing thrombocytosis. Considering the potential for his peripherally inserted central catheter line to clot during this thromboembolic illness, hematology was consulted and agreed he should be treated with enoxaparin. On discharge, an ultrasound demonstrated resolution of the internal jugular thrombus, and his blood cultures were sterile. He was discharged on hospital day 13. He completed outpatient parenteral antibiotic therapy through a peripherally inserted central catheter with 24 days of ertapenem and enoxaparin. He had frequent follow‐up with pediatric infectious disease and made a complete recovery. 2 DISCUSSIONLemierre's syndrome is a rare disease that is estimated to have a worldwide incidence of 1/1,000,000.1 It occurs when an oropharyngeal infection spreads into deep spaces within the neck, causing septic thrombophlebitis and possible spreading septicemia if not caught early.2, 3 Typically, F. necrophorum is involved, causing a bloodstream infection and promoting the formation of septic emboli, which can migrate to pulmonary capillaries1, 4, 5 Typical treatment for Lemierre's syndrome involves intravenous antibiotics to treat the underlying pathogens. The use of anticoagulation medication, such as low‐molecular weight heparin, is considered controversial, as limited research has been done with regard to its effectiveness in Lemierre's syndrome.4, 6 This patient was evaluated in the midst of a COVID‐19 pandemic in a geographic area that was greatly affected by the high prevalence of SARS‐CoV‐2. Despite a sore throat, he was triaged into an ED pod for cohorted patients with suspected COVID‐19. This initial decision could potentially have led to further cognitive errors on the part of the treatment team. Putting too much weight on this initial presentation of fever in a pandemic would have been consistent with "anchoring bias" in which initial facts distort the clinician's ability to take in further information objectively.7 It has been well established that clinicians who succumb to anchoring effects are more likely to make diagnostic errors.8-11 Despite the patient's initial findings, some of which could have been consistent with the frequently encountered clinical deterioration with acute COVID‐19, the ED team recognized alternative diagnoses for his persistent fevers, tachycardia, and hypotension and appropriately treated his septic shock with broad‐spectrum antibiotics and fluid resuscitation. Although our case is similar to the characteristic clinical picture of Lemierre's syndrome resulting from strep pharyngitis, the diagnosis was complicated by a group G streptococcus infection. The rapid antigen detection testing is highly specific for group A strep, but does not reliably identify group G strep.12 This case is further complicated by the lack of data concerning the reliability of SARS‐CoV‐2 viral testing. This patient was tested multiple times and suspicion remained high even after negative results. The sensitivity of this testing varies by type of specimen obtained, and there is a lack of gold standard testing for comparison. The reported sensitivity of nasopharyngeal specimens appears to vary between studies, all with relatively few participants. A discussion by Woloshin et al13 in the New England Journal of Medicine notes that a preprint study from Wuhan, China, found that among 490 nasal swabs from 213 hospitalized patients with COVID‐19, 27% were falsely negative. A systematic review by Arevalo‐Rodriguez et al14 suggested that the false negative rate could be between 2%–29%, equating to 71%–98% sensitivity. Because there are still many unknown factors, such as variations between time of testing and stage of disease, a negative COVID‐19 result currently does not reliably exclude the disease. 3 CONCLUSIONThis case report summarized the presentation and treatment of a 17‐year‐old male who was ultimately diagnosed with Lemierre's syndrome days after being treated as a presumed COVID‐19 case. Although he was successfully treated for Lemierre's syndrome, this case illustrates the importance of exploring various diagnoses even when symptoms are consistent with the disease of a current global pandemic. ACKNOWLEDGMENTSWe acknowledge Marna Rayl Greenberg for formatting and editing. The article publishing fees for this manuscript were generously provided by a nonprofit community foundation, the Dorothy Rider Pool Health Care Trust Awards for Clinical Transformational Excellence. CONFLICTS OF INTERESTThe authors have no additional outside support information, conflicts, or financial interest to disclose.
REFERENCES
|
| Posted: 29 Sep 2020 11:18 AM PDT Structural analysis of SARS-CoV-2 mRNA cappingThe development of antiviral drugs that impair SARS-CoV-2 replication or prevent it from evading the host immune system is critical for fighting the COVID-19 pandemic. The methyltransferase that caps viral mRNAs is a potential target for antiviral therapies because capping both enhances the translation of viral proteins and prevents viral mRNAs from being recognized by the host immune system. Rosas-Lemus et al. solved crystal structures for the SARS-CoV-2 methyltransferase, the nsp16-nsp10 heterodimer, in complex with various combinations of its methyl donor and cap structure substrates, a reaction product, and an inhibitor. Together, these structures suggest potential strategies for developing antiviral therapies by disrupting the formation of the active enzyme complex or blocking its catalytic activity. AbstractThere are currently no antiviral therapies specific for SARS-CoV-2, the virus responsible for the global pandemic disease COVID-19. To facilitate structure-based drug design, we conducted an x-ray crystallographic study of the SARS-CoV-2 nsp16-nsp10 2′-O-methyltransferase complex, which methylates Cap-0 viral mRNAs to improve viral protein translation and to avoid host immune detection. We determined the structures for nsp16-nsp10 heterodimers bound to the methyl donor S-adenosylmethionine (SAM), the reaction product S-adenosylhomocysteine (SAH), or the SAH analog sinefungin (SFG). We also solved structures for nsp16-nsp10 in complex with the methylated Cap-0 analog m7GpppA and either SAM or SAH. Comparative analyses between these structures and published structures for nsp16 from other betacoronaviruses revealed flexible loops in open and closed conformations at the m7GpppA-binding pocket. Bound sulfates in several of the structures suggested the location of the ribonucleic acid backbone phosphates in the ribonucleotide-binding groove. Additional nucleotide-binding sites were found on the face of the protein opposite the active site. These various sites and the conserved dimer interface could be exploited for the development of antiviral inhibitors. INTRODUCTIONOn 31 December 2019, the World Health Organization (WHO) was alerted of a pneumonia outbreak with an unknown etiology, originating in the Chinese province of Wuhan, Hubei. The etiological agent was identified as a coronavirus, closely related to the virus responsible for severe acute respiratory syndrome (SARS). The new SARS coronavirus-2 (SARS-CoV-2) causes the severe respiratory infection, coronavirus disease 2019 (COVID-19). Within 4 months, SARS-CoV-2 rapidly spread, sparking a global pandemic. The COVID-19 pandemic has also forced governments to enact "stay-at-home" orders around the world, seriously damaging the global economy (1). According to the WHO, nearly 25 million SARS-CoV-2 infections have been confirmed, of which more than 800,000 were fatal as of 30 August 2020 (www.who.int). These data are similar to those from the Johns Hopkins University tracking system (2). Members of the coronaviridae family of viruses infect birds and mammals, including bats, camels, pigs, and humans. In humans, pathogenic coronaviruses cause acute and severe gastrointestinal infections, fevers, and organ failure. Three of the seven human-tropic coronaviruses—hCoV-229E, hCoV-NL63, and hCoVB-OC43—cause only asymptomatic or mild infections, including the common cold (3). Four other human coronaviruses are linked to severe infections, including hCoV-HKU1, a common cause of pneumonia; SARS-CoV, with a 10% mortality rate; Middle East respiratory syndrome virus (MERS-CoV), with a 37% mortality rate; and SARS-CoV-2, currently with ~3% mortality rate among confirmed cases (3, 4). Among them, SARS-CoV-2 stands as the one with highest transmissibility, making its containment very difficult (5). As SARS-CoV-2 continues to spread, the need for effective vaccines and therapeutics increases. Therefore, it is urgent to study SARS-CoV-2 mechanisms of infection and replication to find effective targets for drug and vaccine development. Coronaviruses have a large (~30 kb) single-stranded, positive RNA genome that is 5′-capped and contains a 3′-poly-A tail. The orf1a and orf1ab coding regions are directly translated, whereas the rest of the genome serves as template to generate subgenomic mRNAs transcribed from the 3′ end, which are later capped and translated (6–8). The first open reading frame (orf1a) produces the large nonstructural polyprotein 1a (pp1a), and a programmed −1 ribosomal frameshift results in translation of the larger nonstructural polyprotein 1ab (pp1ab) from the reading frame orf1ab. These polyproteins are subsequently processed into 16 nonstructural proteins (nsp1 to nsp16) that assemble to form the replication-transcription complex (RTC) or function as accessory proteins necessary for viral replication (8, 9). The components of the RTC include enzymes that regulate mRNA and genomic RNA synthesis, proofreading, and mRNA maturation. Two of these enzymes, nsp14 and nsp16, are critical for capping viral mRNAs, a tactic used by multiple RNA viruses to avoid immune detection (10). In eukaryotic cells, mRNA capping is initiated by an RNA triphosphatase (TPase), which removes the γ-phosphate from the 5′-end of the nascent mRNA transcript, generating a diphosphate 5′-ppN end. An RNA guanylyltransferase (GTase) subsequently catalyzes the hydrolysis of pyrophosphate (PPi) from a guanidine triphosphate (GTP) molecule, thus forming guanidine monophosphate (GMP). This is followed by the transfer of the α-phosphate of GMP to the diphosphate 5′-ppN transcript end, thus forming the cap core structure, methylguanine-triphosphate-ribonucleotide, referred to as GpppN. GpppN formation is followed by N7-methylation of the capping guanylate by a guanine-N7-methyltransferase (N7-MTase) to irreversibly generate the methylated Cap-0. Further methylation at the ribose 2′-O position of the first nucleotide of the RNA is catalyzed by a ribose 2′-O-MTase to generate Cap-1 and sometimes at the second nucleotide to generate Cap-2. Both the N7-MTase and 2′-O-MTase use S-adenosyl-l-methionine (SAM) as the methyl group donor (4, 10). For coronavirus mRNA maturation, the TPase activity is mediated by nsp13 (6, 11–13), and a still-elusive GTase guanylates the 5′-end of the nascent mRNA. The viral nsp14, which has N7-MTase activity, then generates Cap-0 (14). Nsp14 is a bifunctional enzyme with independent N7-MTase and exonuclease domains (15). The association of nsp14 with viral nsp10 specifically stimulates nsp14 exonuclease activity but has no effect on the N7-MTase activity (16). The coronavirus mRNAs are further modified to have a Cap-1 by the viral 2′-O-MTase (nsp16). Nsp16 is a 7-methylguanine-triphosphate-adenosine (m7GpppA)–specific, SAM-dependent 2′-O-MTase (17, 18) that is activated upon binding to nsp10 (16, 19). Nsp10 is a stable monomeric protein that can also form dodecamers (20, 21) in addition to binding to nsp14 and nsp16 (16, 22). Although no specific enzymatic activity has been identified for nsp10, it is known that nsp10 is a zinc-binding protein and can bind RNA (20, 23, 24). It has also been found that nsp10 interacts with human adaptor protein complex 2 when expressed in mammalian cells (25). However, the main known function of nsp10 is the stabilization of the SAM-binding pockets of nsp16 and nsp14 (19). The 2′-O-methylation of coronavirus RNA that is mediated by the nsp16-nsp10 heterodimer is essential for preventing recognition by the host to evade immune responses that are triggered by viral mRNAs (17). Structures of the nsp16-nsp10 complex have been determined for SARS-CoV and MERS-CoV (18, 23, 26, 27), and the analyses elucidated the structural basis for substrate binding and the proposed SN2 mechanism of methyl transfer. To facilitate structure-based inhibitor design, we initiated a project to determine the structures of the 2′-O-MTase from SARS-CoV-2 in complex with its ligands. Here, we present a comprehensive x-ray crystallographic study of the structure of the SARS-CoV-2 nsp16-nsp10 heterodimer. The structures of the heterodimer were determined in complex with the methyl donor SAM, the product of the reaction [S-adenosylhomocysteine (SAH)], and pan-MTase inhibitor sinefungin (SFG). In addition, we describe the first publicly deposited SARS structures of nsp16-nsp10 in complex with the mRNA cap m7GpppA, which facilitates detailed analysis of the changes in the conformation of flexible loops of nsp16 upon substrate binding. Furthermore, we report crystal structures with sulfate ions in the proposed RNA binding groove as well as several additional nucleotide and sugar binding sites outside the active site. Because nsp16 is one of the most conserved proteins of SARS-CoV-2 and related viruses, these high-resolution structures are expected to be useful as models for developing new antiviral therapeutics to treat COVID-19 and other diseases caused by coronaviruses. RESULTSHigh-resolution structures of the SARS-CoV-2 2′-O-MTase heterodimer in two crystal formsThe SARS-CoV-2 proteins nsp10 and nsp16 are encoded by the polycistronic orf1ab of the (+) single-stranded RNA (ssRNA) (Fig. 1A) and are released from the polyproteins pp1a and pp1ab by the protease nsp5 (28). The protein nsp10 is a 14.8-kDa protein released from both polyproteins pp1a and pp1ab, whereas nsp16 is a 33.3-kDa protein released only from the pp1ab polyprotein, which is created by a −1 ribosome shift (Fig. 1A) (28). We successfully expressed and purified recombinant nsp16 and nsp10 separately, combined them 1:1 in the presence of SAM to form nsp16-nsp10 complexes that were set up for crystallization, and obtained diffraction-quality crystals under several conditions (table S1). (A) Linear schematic of the orf1a/orf1b protein product pp1ab before proteolytic processing. (B) Cartoon representation of the nsp16-nsp10 heterodimer of the small unit cell crystal form (PDB code 6W4H). (C) Cartoon representation of the two nsp16-nsp10 heterodimers in the asymmetric unit of the large unit cell crystal form (PDB code 6W75). In (B) and (C), nsp16 is in shades of tan and yellow, and nsp10 is in shades of teal and purple. Ligands are represented as sticks, with SAM in bright green and Zn2+ in purple. N-ter, N terminus; C-ter, C terminus. (D) Elution profile for analytical size exclusion chromatography (SEC) with corresponding plot for molecular weight standards shown at the top. (E) Separation of elution fractions on a 4 to 15% gradient SDS–polyacrylamide gel electrophoresis (PAGE) gel stained with Coomassie blue. Mr, relative molecular mass; Mw, molecular weight. The first structure of the nsp16-nsp10 complex from SARS-CoV-2 was solved at 1.8 Å [Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank (PDB) code 6W4H; Fig. 1B and table S1]. The crystal belonged to the space group P3121 with two polypeptide chains in the asymmetric unit, with chain A (nsp16) and chain B (nsp10) forming a heterodimer. We refer to this crystal form as the "small unit cell." The heterodimer had a total solvent-exposed surface area of 19,710 Å2 and a buried area of 2870 Å2 estimated by the Protein, Interfaces, Structures, and Assemblies tool (PISA), and it is mainly stabilized by hydrophobic interactions and hydrogen bonds at the interface of nsp16 and nsp10. In this structure, the methyl donor SAM was bound to nsp16, and Zn2+ was bound to nsp10 (Fig. 1B). The second crystal form of the nsp16-nsp10 complex with SAM yielded a structure solved at 1.95 Å (PDB code 6W75, table S2). This crystal form belonged to the P3221 space group and had four chains in the asymmetric unit. The four chains were arranged as a dimer of dimers with a butterfly-like shape (Fig. 1C). The two heterodimers interacted by the C terminus of nsp16 and the N terminus of nsp10. We refer to this crystal form as the "large unit cell." The overall structure of nsp16-nsp10 in the large unit cell was almost identical to the small unit cell structure, including the bound ligands SAM and Zn2+. To corroborate the degree of structural identity, the chains of both crystal forms were aligned using the FATCAT server (29). Alignment of nsp16 from the large unit cell (chains A and C) with that from the small unit cell (chain A) showed substantial similarity with a raw root-mean-square-deviation (RMSD) of 0.37 and 0.42 Å, respectively. The introduction of a flexibility factor in the alignment showed an optimized RMSD of 0.38 Å for chain A and 0.77 Å for chain C, demonstrating that the nsp16 structures in these two crystal forms are similar but have flexible regions. One of these flexible regions was a part of the loop formed by the residues Asp6931-Phe6947, which was disordered from the residues Lys6933 to Lys6939 in chain C, a likely explanation for the structural differences (movie S1). The nsp10 alignment had a raw RMSD of 0.23 Å for chain B and 0.34 Å for chain D, and there were no gaps in the alignment. The optimized RMSD was 0.28 and 0.35 Å, respectively, indicating very low differences between these chains. Thus, both crystallographic forms were almost identical with small discrepancies caused by different conformations in the flexible loops of nsp16. To determine which stoichiometry existed in solution, we performed analytical size exclusion chromatography (SEC). In the elution profile, we observed a prominent elution peak at 15 ml that corresponded to a molecular weight of 45 kDa, which is close to the estimated molecular weight of the heterodimer (49.8 kDa). We also observed a small peak at ~17.5 ml, containing mostly nsp10 (Fig. 1, D and E). No peak was detected corresponding to ~90 kDa, which would be consistent with four chains in a complex in solution. Thus, the heterodimer is the most soluble and stable form of the 2-O-MTase complex, and the dimer of dimers in the large unit cell formed as a result of crystal packing. Topology of nsp16 and nsp10 and the heterodimer interfaceThe nsp16 protein consisted of pp1ab residues 6799 to 7096 plus three additional residues (Ser-Asp-Ala) at the N terminus derived from the recombinant expression tag after tobacco etch virus (TEV) (20) protease cleavage. The 2′-O-MTase catalytic core was composed of a Rossmann-like β-sheet fold with the canonical 3-2-1-4-5-7-6 arrangement, in which β7 was the only antiparallel strand (Fig. 2A). This β-sheet was sandwiched by 11 α-helices and 20 loops (Fig. 2B). (A to C) Cartoon representations of two views of nsp16 featuring the canonical β-sheet (A) and overall secondary structure of nsp16 (B) and nsp10 (C). α-Helices are shown as red cylinders, β-strands as yellow arrows, loops as green strands, and zinc ions as purple spheres. (D) Close-up view of the two Zn2+ binding sites in nsp10. (E) Interaction of Cys4294-Leu4298 (sequence CVKML, gray sticks) from nsp10 with the hydrophobic surface of nsp16 (colored by electrostatic potential). Oxygen, red sticks; nitrogen, blue sticks; sulfur, yellow sticks. (F) Schematic representation of residues from nsp16 (blue squares) and nsp10 (tan triangles) that interact through hydrogen bonds, represented as lines. Some interactions are mediated by water molecule (cyan circles). For (A) to (D), structural representations are based on the structure of the nsp16-nsp10 complex with m7GpppA and SAM (PDB code 6WVN). (E) and (F) are based on the structure of the nsp16-nsp10 complex with SAM in the small unit crystal form (PDB code 6W4H). The nsp10 protein, consisting of pp1a residues 4272 to 4392, has at its core three β-strands (β′1, β′2, and β′3) that form a central antiparallel β-sheet. At one side of the β-sheet is the large loop that directly interacts with nsp16 and stabilizes the heterodimer complex. At the other side of this β-sheet, there are six helices and loops that form two zinc fingers (Fig. 2C). In other coronaviruses, these zinc fingers are involved in nonspecific binding of RNA (23, 24). The Zn2+-binding site 1 is coordinated by the residues Cys4327, Cys4330, Cys4336, and His4343. The Zn2+-binding site 2 is coordinated by Cys4370, Cys4373, Cys4381, and Cys4383 (Fig. 2D). The residues forming the nsp16-nsp10 heterodimer interface can be divided into clusters. The clusters for nsp16 are defined as A (residues 6835 to 6846), B (6874 to 6889), C (6900 to 6908), and D (7042 to 7046). For nsp10, they are defined as clusters I (4293 to 4300), II (4322 to 4337), and III (4346 to 4349) (18, 23). Almost all of the interface contacts between nsp16 and nsp10 are formed by hydrophobic interactions between cluster I (Val4295, Met4297, and Leu4298) of nsp10 and cluster A (Pro6835, Ile6838, Met6839, Val6842, and Ala6843), cluster B (Val6876, Pro6878, and Ala6881), and cluster D (Leu7042 and Met7045) of nsp16 (Fig. 2E). We observed that the remaining interactions at the interface are mediated by hydrogen bonds, and these hydrophilic interactions consisted of five direct contacts between residues Lys4296, Leu4298, Ala4324, Tyr4349, and Gly4347 of nsp10 with Lys6836, Gln6875, Ala6881, and Asp6904 of nsp16, respectively, plus eight water-mediated interactions (Fig. 2F). The binding of SAM, SAH, and the inhibitor SFG to the methyl donor–binding siteThe nsp16 protein catalyzes the transfer of the methyl group from SAM to Cap-0, generating the reaction products SAH and Cap-1. This reaction can be inhibited by SFG, a 5′-aminoalkyl analog of SAH used as a pan-inhibitor of MTases (Fig. 3A). To identify potential structural differences caused by having SAM, SAH, or SFG in the SAM-binding cleft, we also determined the structures of nsp16-nsp10 in complex with SAH (PDB code 6WKQ) and SFG (PDB code 6WJT) at 2.0- and 1.98-Å resolution, respectively. These structures showed that SAM binds to a negatively charged cleft formed by αA, αz, αD, and the loops L5, L8, and L11 in nsp16 (Fig. 3B). The adenosine moiety is stabilized by residues Phe6947, Asp6912, Leu6898, Cys6913, and Met6928. The sugar moiety is stabilized by the residues Gly6871 and Asp6897 and by two molecules of water that interact with Asn6899. The methionine moiety interacts with Asp6928, Tyr6845, Asn6841, and Gly6871. SAH and SFG interact with the same residues as SAM without modifications in the site or the overall structure (Fig. 3C). (A) Chemical structures of the methyl donor SAM; the product after methyl transfer, SAH; the SAH analog and inhibitor, SFG; and the Cap-0 analog, m7GpppA. Boxes highlight differences in the chemical structures of SAH and SFG compared to SAM. (B) Cartoon and surface charge representations of the nsp16 SAM-binding cleft occupied by SAM (green sticks). (C) Close-up view of the overlay of nsp16 structures with the ligands SAM (green, PDB code 6W75), SAH (pink, PDB code 6WJT), and SFG (orange, PDB code 6WQK). (D) Cartoon and surface charge representations of nsp16 (PDB code 6WVN) with the SAM-binding cleft occupied by SAM (green sticks) and the Cap binding site occupied by m7GpppA (gray sticks). (E) Detailed view of the residues that coordinate m7GpppA in the Cap binding site as tan sticks. (F) Close-up view of the side chains of the catalytic residues showing the orientation of the methyl group in SAM in proximity to the acceptor 2′-OH group in m7GpppA. Dashed lines indicate the interactions between the residues in the active site, and small cyan dots indicate water molecules (w). Red sticks, oxygen; blue sticks, nitrogen; orange sticks, phosphate; yellow sticks, sulfur. Comparison of nsp16-nsp10 from SARS-CoV-2 and SARS-CoVAt the primary amino acid sequence level, nsp16 from SARS-CoV-2 is 99% identical to Bat-CoV-RaTG13, 94% identical to Bat-SARS–like coronavirus Rs4247(Bat-SL-CoV), and 93% identical to SARS-CoV, but only 66% identical to MERS-CoV (fig. S1A). The primary amino acid sequence of nsp10 is 100% identical to Bat-CoV-RaTG13, 99% identical to SARS-CoV, and 98% identical to Bat-SL-CoV, but only 59% identical to MERS-CoV (fig. S1B). Thus, nsp16 and nsp10 are highly conserved in the lineage B betacoronaviruses (Bat-SL-CoV, Bat-CoV-RaTG13, and SARS-CoV), and less conserved with lineage C betacoronaviruses (MERS-CoV). We compared our structures from SARS-CoV-2 with published structures from SARS-CoV (18, 23) to determine whether minor differences in sequence affected the structures of nsp16 or nsp10. The two amino acid differences in nsp10 between SARS-CoV-2 and SARS-CoV are A4276P and K4366R and do not introduce important structural changes (fig. S1C). Furthermore, in the aligned sequences, the Zn2+-coordinating residues in nsp10 are 100% conserved, emphasizing the importance of the zinc fingers across the coronaviruses (fig. S1B). Another difference was identified when our nsp16 structure was aligned with the SARS-CoV nsp16 structure published by Chen et al. (23), which has three gaps and an optimized RMSD of 1.19 Å. The differences were localized to loop 1 and helix η3 of SARS-CoV-2 (fig. S1B). The loop 1 sequence that starts at residue 6829 is DSATL in SARS-CoV-2, compared to ENAVI in SARS-CoV. This loop is flexible and is in a "closed" conformation in SARS-CoV compared with a more "open" conformation for SARS-CoV-2. In addition, η3 in SARS-CoV-2 is modeled as a loop in SARS-CoV, which may be due to differences in the primary sequence of PKTKN compared to PRTKH. All other amino acid differences were solvent-exposed (fig. S1C), and none of them affected the SAM-binding site. Further, the nsp16-nsp10 interface residues identified for SARS-CoV-2 (Fig. 2, E and F) are 100% identical to this interface in SARS-CoV (fig. S1, A and B), indicating that the interface is conserved between the two viruses. Despite the sequence divergence, the overall structure of the SARS-CoV-2 2′-O-MTase is very similar to structures available for the MERS-CoV 2′-O-MTase (27), with no substantial changes in nsp10 (RMSD = 0.78 Å). The MERS-CoV nsp16 structure is also very conserved, but the loops L1 and L8–L9 are disordered, which causes an increase in the RMSD of 0.69 Å. One difference between the SARS-CoV-2 and MERS-CoV 2′-O-MTases exists at the nsp16-nsp10 interface. Tyr4349 in nsp10 contacts Ala6881 in nsp16, two residues that are conserved in SARS-CoV-2, and all other betacoronaviruses. However, in MERS-CoV, the corresponding residues are Phe and Ser, respectively (fig. S1, A and B). This Phe substitution in MERS-CoV nsp10 promotes the binding of nsp10 to nsp16 and stimulates the MTase activity of nsp16 (30). The conservation of Tyr at this position in SARS-CoV-2 and SARS-CoV could indicate a positive selection against overstimulation of the MTase in the lineage B betacoronaviruses. Binding of SAM and m7GpppA to the nsp16 catalytic siteIn addition to the methyl donor SAM, the nsp16 catalytic reaction requires a Cap-0 mRNA with an adenosine in position 1 of the ssRNA (m7GpppA-RNA). The crystal structure of the ternary complex of nsp16-nsp10 with m7GpppA and SAM has been determined for the more distantly related lineage C betacoronavirus MERS-CoV [PDB code 5YNM (27)], but not for the more closely related lineage B betacoronavirus SARS-CoV, in which the binding of the mRNA was previously only modeled (23). Here, we describe structures of the SARS-CoV-2 nsp16-nsp10 heterodimer in complex with m7GpppA and either the methyl donor SAM (PDB code 6WVN) or the product SAH (PDB codes 6WQ3 and 6WRZ). Overall, the three were almost identical between the SAM- and SAH-bound structures (RMSD of 0.12 Å) except for sulfates bound into the RNA binding groove at different positions in one of the structures with SAH. One of the structures with cap and SAM (PDB code 6WVN) was selected for more detailed analysis because it was the most complete, with eight additional residues at the N terminus of nsp10 compared to the SAM-bound structure (PDB code 6W4H), and is at slightly better resolution (table S2). The N terminus in this structure forms an α-helix that seems to be in a more open conformation with respect to the previous structure from MERS (RMSD = 0.84 Å) (27). The cap analog m7GpppA bound to the cap high-affinity binding site (HBS), which is a positively charged surface on nsp16 formed by the loops L1, L8, L9, L10, and L12, αD, and the η3 (Fig. 3D). The guanosine ring of m7GpppA is stacked with Tyr6828. The phosphate groups were mostly stabilized by side-chain atoms of Tyr6828, Tyr6930, Lys6935, Thr6970, Ser6999, and Ser7000 and by the main-chain atoms of His6972 and Ser7000 of loops 10 (residues 6970 to 6975) and 12 (residues 6994 to 6997; Fig. 3E). The adenosine sugar interacted with side-chain atoms of Lys6844 and Lys6968 and with Asp6928 through a water molecule. The adenine moiety was stabilized by stacking interaction with the side chain of Tyr6930 (Fig. 3F), and it was in close proximity to the SAM-binding cleft. These interactions are also found in the structure of MERS-CoV nsp16 in complex with Cap-0 [PDB code 5YNM (27)]. The high quality of the crystal structure of the nsp16-nsp10 complex with m7GpppA and SAM bound facilitated detailed analysis of the catalytic site. The protein nsp16 contains the highly conserved residues Lys6839, Asp6928, Lys6968, and Glu7001, comprising the canonical catalytic motif (K-D-K-E) conserved among class I MTases (18, 23, 31). These residues are close to the SAM methyl group that is transferred to the 2′-OH on m7GpppA. The amino group of Lys6968 is in close interaction with the 2′-OH of Cap-0 and possibly activates this oxygen for the nucleophilic attack of the methyl group in SAM (Fig. 3F). In the structure of nsp16-nsp10 with m7GpppA and SAM, we detected the presence of one molecule of water (Fig. 3F) that might participate in the stabilization of intermediate catalytic states (18, 23, 32). Although the nsp16 MTase reaction was previously characterized as Mg2+-dependent, we did not observe this metal bound, and it is likely that Mg2+ is involved only in transitory states of catalysis or stability of the protein as previously suggested for dengue virus 2′-O-MTase (33). The flexibility of the m7GpppA site in nsp16Previous biochemical studies of SARS-CoV nsp16 demonstrated stabilization of the SAM cleft upon nsp10 binding (18, 23) and improved m7GpppA-RNA interaction (23), although there is as yet no structure with cap bound to SARS-CoV nsp16 to identify the conformational changes necessary for cap binding. The HBS is surrounded by loop 1 (residues 6824 to 6834) and a loop formed by L8–η-3–L9 (residues 6930 to 6943) in nsp16. These flexible loops, which were not visible in the previously published structure for MERS, are visible in our structures, and thus, we could analyze the diverse conformations of nsp16-nsp10 complexes. First, we compared the structures of the heterodimer with only SAM bound from the large unit cell crystal form (Fig. 4A, green) with that from the small unit cell crystal form (Fig. 4A, blue). There are only minor conformational differences for loop 1 of nsp16; however, the L8–η-3–L9 loop showed a more open conformation in the large unit cell and a more closed conformation for the small unit cell structure when only SAM was bound. This analysis corroborated that this specific region is flexible and that these loops likely transit from an open state to a closed state in the absence of m7GpppA. (A) Alignment of the C-α chain of nsp16 from SARS-CoV-2 in complex with SAM from the small unit cell (blue, PDB ID 6W4H), in complex with SAM from the large unit cell crystal form (green, PDB code 6W75), and in complex with SAM and m7GpppA (orange, PDB code 6WVN). Two flexible loops are enlarged in insets. (B) Alignment of the C-α chain of nsp16 from SARS-CoV-2 in complex with SAM and m7GpppA (orange) with the corresponding region of MERS-CoV nsp16 in complex with SAM alone (light blue, PDB code 5YN6) or in complex with SAM and m7GpppA (cyan, PDB code 5YNM). Numbering of MERS residues is indicated in parentheses. To evaluate the position of the loops when m7GpppA is bound, we analyzed the structural alignment between the high-resolution heterodimer with SAM bound (Fig. 4A, blue) and the heterodimer in complex with m7GpppA and SAM (Fig. 4A, orange). This alignment has an RMSD of 0.55 Å, and we observed that the presence of m7GpppA induces a stable open conformation of L8–η-3–L9 (residues 6930 to 6943), which was found also in the heterodimer structures in complex with m7GpppA and SAH (RMSD = 0.12 Å; PDB codes 6WQ3 and 6WRZ). The main conformational changes were observed near residues 6936 to 6939, which were displaced in the open conformation and appeared to mitigate clashes between Lys6935 of nsp16 with the ribose and the first phosphate group of m7GpppA and at residue Tyr6930 that is rotated to improve stacking interaction with the adenine of Cap-0 (fig. S2). These changes indicated that the presence of m7GpppA stabilized an open state of the Cap-0 binding site, which could facilitate the release of the product upon methylation. Furthermore, the C-α chain of nsp16 from SARS-CoV-2 with Cap-0 and SAM bound (Fig. 4B, orange) was aligned with MERS-CoV nsp16 with SAM (Fig. 4B, violet) or with Cap-0 and SAM bound (Fig. 4B, cyan) over 293 residues. In the absence of Cap-0, the MERS-CoV nsp16 residues corresponding to residues 6934 to 6940 in SARS-CoV-2 were disordered, confirming that this loop is flexible. In contrast, loop 1 and residues 6930 to 6943 from the MERS-CoV m7GpppA-nsp16 complex align with the open conformation observed in the SARS-CoV-2 m7GpppA-nsp16 complex. Sulfates align to the RNA binding grooveThe 2′-O-MTase nsp16-nsp10 possibly binds the viral RNA in the positively charged nucleotide-binding groove, also known as the low-affinity binding site (LBS). There is, as yet, no structural evidence of the arrangement of this part of the viral RNA in the protein, and only predictive models have thus far been published (23, 34, 35). Sulfates are known to bind to proteins in the same positions as nucleic acid backbone phosphates and thus can be used to model nucleic acid binding regions. Because the first small unit cell structure (PDB code 6W4H) was obtained from crystallization conditions with polyethylene glycol (PEG), which did not allow for soaking with substrates, crystals were screened for suitable conditions for soaking with substrates followed by cryoprotection with 2 M lithium sulfate (see Materials and Methods). We speculated that these sulfate molecules could indicate the possible binding sites for the phosphates of the RNA molecule. All of the structures with m7GpppA also had molecules of sulfates bound at distinct sites and could be superimposed to analyze the position of sulfates (Fig. 5, A to C). Sulfate 1 (S1) was in a position next to the SAM cleft in two alternative conformations, which could indicate the importance of charged molecules near the catalytic site (Fig. 5B). Sulfate 2 (S2) seems to mimic the phosphate group between the first and second nucleotide in the RNA and is followed by a zigzag line of other sulfates (S2 to S5) along the positively charged LBS from nsp16 and the extension groove of nsp10. The compilation of these results suggested that the nucleotide-binding groove might accommodate four to five nucleotides from the viral m7GpppA-RNA (Fig. 5B). This experimental and structural evidence reveals the possible position of the viral RNA in the nucleotide-binding groove. (A) Surface charge representation of nsp16-nsp10 bound to SAM and m7GpppA (PDB code 6 WVN) with sulfates in balls and sticks along the nucleotide-binding groove numbered from the catalytic core to the nsp10 extension (S1 to S5). The sulfates in the overlayed structures are designated by color according to their corresponding PDB code: 6WRZ (green), 6WVN (yellow), and 6WQ3 (pink). m7GpppA is shown as gray sticks, and SAM is shown in green sticks. Positive charges are shown in blue, and negative charges are shown in red. ADE2, adenine moiety 2. (B) Close-up view of (A) showing m7GpppA, SAM, and S1 to S5 in the high-affinity binding site (HBS) and low-affinity binding site (LBS). (C) A 90° rotation of the complex showing the secondary binding sites MGP and ADE1 along with additional sulfates. (D) Schematic representation of m7GpppA noting the MGP (m7GpppA guanine and phosphate) and ADE (adenine) moieties. (E) Surface charge representation of the nsp16 MGP binding site with MGP (yellow sticks) and BDF (pink sticks) from structure 6W4H. (F and G) Cartoon and surface charge representation of the adenine moieties (ADE1 and ADE2) bound to nsp16 from structure (PDB code 6WVN). Sticks, carbon; blue, nitrogen; red, oxygen; orange, phosphate; yellow, sulfate. Identification of three additional m7GpppA-binding sites in nsp16The present comprehensive study of the interaction of m7GpppA with nsp16-nsp10 resulted in the unexpected finding of nucleotides in noncatalytic sites of the structures (Fig. 5, A and C). Although relatively short soaking times were tested to avoid nonspecific binding of m7GpppA, nucleotides were consistently found at three different positions in addition to the active site. One of the sites showed binding of the guanine and phosphate moiety from m7GpppA (designated MGP; Fig. 5D) and is located on face of the protein opposite the active site (Fig. 5C). This site was found occupied by a guanine moiety in all three structures with m7GpppA. The guanine moiety of m7GpppA interacts with the hydrophobic surface formed by Trp6987 and with the NH2 in position 2 stabilized by Ser7074 in a small negatively charged cavity (Fig. 5E). The adenosine moiety of the m7GpppA ligand was disordered. In the structure without the cap (PDB code 6W4H), this same site was occupied by β-d-fructopyranose (BDF) (Figs. 1B and 5E), indicating that this site is not nucleotide-specific. Another binding site (ADE1) was occupied by an adenine (ADE) moiety that likewise is derived from m7GpppA (Fig. 5, C and D). The ADE stacked with Trp6803 (Fig. 5F). The third binding site (ADE2) is also occupied by an adenine ring stacking with Tyr7020 (Fig. 5, A and G). Although these noncatalytic nucleotide-binding events might be crystallization artefacts, they may represent interactions that would mimic the interaction between nsp16 and the ribonucleotides of the capped mRNA. DISCUSSIONThe SARS-CoV-2 pandemic has yielded an urgent worldwide effort to understand the molecular mechanisms involved in coronavirus transmission, virulence, and replication (1). The ultimate goal is to identify viral proteins that are amenable to drug targeting and epitopes suitable for vaccine development. Although previous studies conducted in the related betacoronaviruses SARS-CoV and MERS-CoV paved the way for drug discovery and vaccine development, no approved treatments were fully developed (36). Thus, to ensure an accurate approach for drug discovery, we present a comprehensive study of the first structures of the SARS-CoV-2 2′-O-MTase complex that were publicly available to the scientific community. In addition to the structures reported here, similar structures of the nsp16-nsp10 complex have subsequently been determined by other groups including structures with SAM [PDB codes 6W61 (37), 7BQ7 (38), 7C2I, and 7C2J (39)] and with SFG [PDB code 6YZ1 (34)]. Independently, another structure of nsp16-nsp10 in complex with m7GpppA + SAM was also deposited [PDB code 6WKS (35)] but released subsequent to our structure (PDB code 6WQ3). Previous studies determined that the 2′-O-MTases of SARS-CoV and MERS-CoV, which share 93 to 99% and 59 to 66% identity to SARS-CoV-2 MTase, respectively, are heterodimers formed by the binding of nsp10 to nsp16 (16, 22). In addition, their structures have provided some insight into nsp10-dependent activation of nsp16 MTase catalysis (18, 23, 27). Because variation at the primary sequence level can affect both local and overall structure, ligand binding and structure-based drug design can also be affected by small changes in amino acid sequence. Thus, high-resolution structures of the 2′-O-MTase from SARS-CoV-2 are necessary to best inform drug discovery for COVID-19. The present study demonstrated that the binding site for the methyl donor SAM was highly conserved, especially the canonical Gly-X-Gly motif located at the end of the β1 and αA and Phe6949, as found in almost all class I MTases (40, 41). SAM analogs have been proposed as antimicrobials targeting MTases of fungi and parasites (42, 43). Here, we determined the structure of nsp16-nsp10 bound to the pan-MTase inhibitor SFG and showed that SFG has nearly identical interactions with amino acid side chains as the natural substrate SAM. The high resolution of the structures with bound SAM, SAH, and SFG could facilitate the computational design of small molecules that have higher affinity than SAM for the SAM-binding cleft and have specificity for SARS-CoV-2 nsp16 compared to host MTases. Further, the conservation of the binding cleft residues across the betacoronaviruses (fig. S1) suggests that an inhibitor designed for SARS-CoV-2 could also be a broader spectrum inhibitor, which could target other coronavirus 2′-O-MTases. The Cap-0 binding site offers another position in nsp16 that could be a target for small-molecule inhibition. Although the published structure of nsp16-nsp10 from SARS-CoV was determined with SAM bound, only a computational model of the interaction of SARS-CoV nsp16 with m7GpppA-RNA was previously available (23). To study the different arrangements in this structure upon Cap binding, crystals were soaked with m7GpppA in the presence of both SAM and SAH. The resulting structures identified the residues that interact with Cap-0 and showed that conformational changes in the Cap-0 binding site could occur during catalysis. Of particular concern for the potential development of a more broad-spectrum inhibitor that could target the Cap binding site are two nearby loops that are variable in sequence across the betacoronaviruses, which could have an impact on catalysis (Fig. 5). Our analysis of overlapped structures of nsp16 from the SARS-CoV-2 nsp16-nsp10 complex with the structure of SARS-CoV nsp16 with the Cap binding site unoccupied demonstrated that these amino acid differences do not affect the overall structure of the complex but rather are highly flexible loops that are then stabilized upon binding of the Cap. Stabilization of these loops may be critical to obtain a high-affinity small-molecule inhibitor directed at this site. The high-resolution structure of SARS-CoV-2 with the cap bound should facilitate the design of such a molecule. A computational model of SARS-CoV 2′-O-MTase in complex with RNA was proposed previously using the structure of vaccinia virus MTase (PDB code 1AV6) as the model (23, 34, 35). This model suggested that Asp75 in SARS CoV (Asp6873 in SARS-CoV-2) confers the selectivity of the Cap binding site for m7GpppA over m7GpppG due to steric hindrance of the Asp6873 residue with the NH2 at position 2 of the guanidyl (23). This residue is conserved in SARS-CoV-2. However, in all our structures with Cap bound, position 2 of the adenylate from m7GpppA is 7 Å away from the oxygen of the Asp6873, suggesting that this residue is unlikely to be involved in the selectivity of the RNA-capped substrate in SARS-CoV-2. Recent studies show that m7GpppG-RNA is 2′-O-methylated by nsp16-nsp10 from SARS-CoV-2 but at a lower efficiency than is m7GpppA-RNA (35, 44), indicating that this site can accommodate m7GpppG. In addition to the ligand binding sites, we explored the positively charged nucleotide groove, or LBS, which leads from the catalytic core of nsp16 toward nsp10. Several efforts to obtain a short RNA bound into this groove of crystals have thus far been unsuccessful, but several computational models have been recently published and are already available for SARS-CoV (23) and SARS-CoV-2 (23, 34, 35). However, no structural evidence of RNA binding has been reported. Thus, to obtain experimental evidence of the possible accommodation of the phosphate groups for four to five ribonucleotides of the mRNA that will directly interact with the nsp16-nsp10 groove, we cryoprotected crystals with lithium sulfate. We speculate that small charged molecules could be designed to prevent binding of mRNA, which could impair the efficiency of the MTase reaction. The potential advantage of targeting a site away from the SAM or Cap binding sites could prevent potential toxicity by avoiding cross-inhibition of human MTases. To this end, our study revealed previously unknown features in the nsp16-nsp10 structure that could be advantageous for the design of new therapeutics. First, adenine was found to bind at two different sites, stacking with Tyr7020 and Trp6803 when the crystal was soaked with m7GpppA. Further, another possible nucleotide-binding site occupied by a guanine moiety was identified on the back of surface of nsp16. This site was also independently identified by another group as occupied by adenosine (35). In addition, we observed BDF in this site when crystals were cryoprotected with sucrose in the absence of m7GpppA, suggesting that this site is nonspecific. Thus, this binding site needs to be further studied to determine whether the binding of other molecules might affect the activity of the enzyme and, if so, whether these newly defined binding sites could be used as potential candidates for developing inhibitors. A final focus for development of inhibitors against nsp16 is to target the interface with its activator nsp10. Peptides derived from nsp10 have been developed to target SARS-CoV nsp16 to impair nsp10 binding to nsp16, resulting in inhibition of nsp16 MTase activity (45). In our analysis, we found that the residues that form the interface between nsp16 and nsp10 are 100% conserved with SARS-CoV. Thus, we predict that small molecules or peptides that target the nsp16-nsp10 interface could also be highly effective inhibitors of nsp16 from SARS-CoV-2 and closely related coronaviruses. An advantage of an inhibitor that instead binds specifically to nsp10 could have even broader implications and potentially also inhibit the N7-MTase nsp14, which is also activated by binding to nsp10 (16, 46). One problem for the development of antiviral compounds against SARS-CoV-2 is the potential impact of emerging mutations in its genome. Newly emerging mutations in SARS-CoV-2 have recently been mapped onto the structures of the proteins they affect (https://coronavirus3d.org) (7). Few mutations have emerged for nsp10, and none of those are predicted to affect the structure of the protein. Some emerging mutations have been identified for nsp16, but likewise, none of these are predicted to disrupt the structure. Further, review of these data showed that no mutations of residues at the interface between nsp16 and nsp10 have yet been detected in SARS-CoV-2 viral variants isolated from around the globe (7). Mutations that affect nsp16 activity may be absent from clinical isolates because a reduction or failure in capping could result in early immune detection and accelerate the clearance of the virus. In support of this, recent studies have suggested that stimulation of an interferon response early in SARS-CoV-2 infection could result in less severe disease, particularly in younger individuals (47). In addition, mice infected with mouse hepatitis virus (MHV) showed improved survival when treated with a 29–amino acid peptide, based on the loops of nsp10, that inhibits nsp16 activation. The protection was correlated with higher levels of interferon during early infection, resulting in lower viral titers (45). Together, this analysis suggests that immunological studies of the impact of nsp16 inhibition could benefit from the identification of an inhibitor that can be used for study of the virus in vitro or in animals. Such an inhibitor could also be used during early infection to stimulate immunity, reducing the likelihood for development of severe disease. The structural work found in this study will help with these next stages in our understanding of MTase-mediated modification of viral mRNA and improved treatments for COVID-19. MATERIALS AND METHODSChemicals and synthetic DNACommon biological chemicals were obtained from MilliporeSigma or Thermo Fisher Scientific unless otherwise indicated. DNA sequences corresponding to the predicted amino acid sequence for nsp10 and nsp16 from SARS-CoV-2 isolate Wuhan-Hu-1 (NC_045512) were codon-optimized for expression in Escherichia coli using GenSmart Codon Optimization followed by manual editing. The genes were synthesized and cloned into the pMCSG53 vector (48) by Twist Biosciences (South San Francisco, CA). The vector sequences add a TEV-cleavable N-terminal 6×His-tag to expressed proteins. The plasmids were transformed into competent E. coli BL21(DE3)(Magic) cells (49). Protein expression, purification, and analytical SECTransformed E. coli cells were cultured for expression in Terrific Broth medium (BD Difco) supplemented with ampicillin (200 μg/ml) and kanamycin (50 μg/ml) incubated at 37°C and 220 rpm. Protein expression was induced at OD600 (optical density at 600 nm) = 1.8 to 2 by an addition of 0.5 mM isopropyl-β-d-thiogalactopyranoside (Research Products International), and the cultures were further incubated at 25°C, at 200 rpm for 14 hours (50). The cells were harvested by centrifugation and resuspended in lysis buffer (50 mM tris, 0.5 M NaCl, 10% glycerol, and 0.1% IGEPAL CA-630) and frozen at −30°C until purification. Frozen suspensions of cells with expressed nsp10 or nsp16 were thawed and sonicated at 50% amplitude, in 5 s × 10 s cycle for 20 min at 4°C. The lysate was cleared by centrifugation at 18,000g for 40 min at 4°C, the supernatants were collected, and the protein was purified as previously described with some modifications (51). Each supernatant was loaded into a His-Trap FF [Ni–nitrilotriacetic acid (NTA)] column using a GE Healthcare ÅKTA Pure system using loading buffer [10 mM tris-HCl (pH 8.3), 500 mM NaCl, 1 mM tris(2-carboxyethyl) phosphine (TCEP), 2 mM MgCl2, and 5% glycerol]. The column was washed with loading buffer, followed by 10 mM tris-HCl (pH 8.3), 500 mM NaCl, and 25 mM imidazole, and the protein was eluted with 10 mM tris (pH 8.3), 500 mM NaCl, and 1 M imidazole. The protein was loaded onto a Superdex 200 26/600 column, ran with loading buffer, collected, and incubated with TEV-protease overnight. The cleaved tag and TEV protease were separated from protein by Ni-NTA affinity chromatography using loading buffer and nsp10 was collected in the flow through, whereas nsp16 was eluted with 50 mM imidazole. To form the nsp16-nsp10 complex, the pure proteins were mixed at a 1:1 molar ratio at about 2 mg/ml in loading buffer, incubated for 1 hour, and then dialyzed in crystallization buffer [10 mM tris-HCl (pH 7.5), 150 mM NaCl, MgCl2, TCEP, and 5% glycerol] for 2 hours (26). SAM was added to a final concentration of 2 mM. The complex was concentrated to 4.5 to 10 mg/ml and set up for crystallization immediately. To confirm that the nsp16-nsp10 complex was formed, we performed analytical SEC using a Superdex 200 10/30 column (GE Healthcare) with 10 mM tris-HCl (pH 7.5), 150 NaCl, 2 mM MgCl2, 1 mM TCEP, and 5% glycerol. The standard calibration curve was obtained using combined low–molecular weight and high–molecular weight Gel Filtration Calibration Kits (GE Healthcare). The resulting peaks from the elution of the protein were fractionated in 0.5 ml. Each fraction was collected, and 8 μl of sample was denatured with Laemmli buffer (Bio-Rad) and then separated using 4 to 15% gradient SDS–polyacrylamide gel electrophoresis (Bio-Rad). Crystallization, soaking, and cryoprotection conditionsThe nsp16-nsp10 complex + SAM was set up as 2-μl crystallization drops (1-μl protein:1-μl reservoir solution) in 96-well (Corning) plates using commercially available Classics II, PEG's II, AmSO4, Anions, and ComPAS Suites (Qiagen). Diffraction quality crystals appeared after 5 to 10 days in 78 conditions, 118 crystals of various complexes were frozen, and 57 datasets were collected. The crystals were soaked, cryoprotected, and flash-frozen for data collection as follows. The small unit cell crystal (6W4H) was cryoprotected with 25% of sucrose in the well solution and the large unit cell crystal (6W75)—with 4 M sodium formate (table S1). To obtain complexes with SAH and SFG, crystals were transferred into the 10-μl drops with their well solutions supplemented with 5 mM SAH or SFG, soaked for 3 hours, cryoprotected with 4 M sodium formate or 2 M LiSO4, and flash-frozen. In an attempt to observe the complexes of nsp16-nsp10 with SAM + m7GpppA and SAH + m7GpppA, crystals were transferred into 10-μl drops containing 5 mM SAM or SAH and 0.5 mM m7GpppA in their respective well solutions, soaked for various amount of time from 3 min to 6 hours, and flash-frozen. LiSO4 (2 M) was used as a cryoprotectant in an attempt to observe the binding of sulfates on the places of phosphates from the RNA in the RNA binding groove. Crystals grown in PEG conditions were found not suitable for these soaks due to a phase separation of m7GpppA in the presence of PEG. Data collection and structure determinationAlmost 120 crystals were screened, and 57 datasets were collected at the Life Sciences–Collaborative Access Team (LS-CAT) beamlines D, G, and F at the Advanced Photon Source (APS) at the Argonne National Laboratory. All the datasets reported here were collected at the beamline F. Images were indexed, integrated, and scaled using HKL-3000 (52). Seven structures were chosen to be described in this manuscript (table S2). The first structure of nsp16-nsp10 from SARS-CoV-2 in complex with SAM with the small unit cell was determined by Molecular Replacement with Phaser (53) from the CCP4 Suite (54) using the crystal structure of the nsp16-nsp10 heterodimer from SARS-CoV as a search model [PDB ID 3R24 (23)]. For all other crystal structures, refined structure from this crystal form was used as a search model. The initial solutions went through several rounds of refinement in REFMAC v. 5.8.0258 (55) and manual model corrections using Coot (56). The water molecules were generated using ARP/wARP (57), SAM, SAH, or SFG; Zn2+ and ligands were added to the model manually during visual inspection in Coot. Translation-Libration-Screw (TLS) groups were created by the TLSMD server (58), and TLS corrections were applied during the final stages of refinement. MolProbity (59) was used for monitoring the quality of the model during refinement and for the final validation of the structure. A total of seven structures were deposited to the PDB (www.rcsb.org/) with the assigned PDB codes 6W4H, 6W75, 6WJT, 6WQK, 6WQ3, 6WVN, and 6WRZ with associated validation reports including electron density maps of all ligands of interest. Sequence and structural alignmentThe protein sequence of nsp16 and nsp10 from SARS-CoV-2 (YP_009725295.1), Bat-CoV-RaTG13 (QHR63299.1), Bat-SL-CoV Rs4247 (ATO98179.1), SARS-CoV-1 (ACZ72252.1), and MERS-CoV (YP_009047238.1) was obtained from the National Center for Biotechnology Information (NCBI) database. The multiple sequence alignment was performed using Clustal-O (www.ebi.ac.uk/Tools/msa/clustalo/) and merged with the coordinates of the structure deposited as PDB code 6w4h using ESPript 3.x (60). The PDB coordinates of SARS-CoV nsp16 and nsp10 were analyzed on the FATCAT (29) and PDBFlex servers (61) to perform structural, flexibility, and sequence alignment. Structural alignments and structure figures were downloaded from the servers and modeled in PyMOL open source V 2.1 (62). The movie showing the flexibility of nsp16 was generated with the files downloaded from PDBFlex server, and frames were captured in PyMOL and exported to iMovie editor. SUPPLEMENTARY MATERIALSstke.sciencemag.org/cgi/content/full/13/651/eabe1202/DC1 Fig. S1. Sequence alignments of nsp16 and nsp10 proteins from betacoronaviruses. Fig. S2. Displacement of the Lys6935 and Tyr6930 upon m7GpppA binding. Table S1. Crystallization, soaking, and cryoprotection conditions. Table S2. Crystallographic data. Movie S1. The flexibility of the Cap binding site of nsp16. REFERENCES AND NOTES
Acknowledgments: We thank L. Jaroszewski and A. Godzik for construct design and G. Wiersum for protein expression. Funding: This project has been funded in whole or in part with Federal funds from the Department of Health and Human Services, NIH, and National Institute of Allergy and Infectious Diseases under contract no. HHSN272201700060C. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (grant 085P1000817). Author contributions: M.R.-L. and O.K. purified proteins and formed the complex. L.S. conducted crystallization and soaking experiments. G.M. and J.B. collected crystallographic data and determined and analyzed structures. M.R.-L. and N.L.I. wrote the draft of the manuscript, which was edited by all authors. K.J.F.S. supervised all aspects of the project. Competing interests: K.J.F.S. has a significant financial interest in Situ Biosciences, LLC, a contract research organization that conducts antimicrobial testing for industrial products including antiviral testing. This work has no overlap with the interests of the company. K.J.F.S. is a consultant for a healthcare firm on public health topics related to SARS-CoV-2 and COVID-19 that are unrelated to this article. The other authors declare that they have no competing interests. Data and materials availability: All data are publicly available in the RCSB PDB (www.rcsb.org) with the PDB codes 6W4H, 6W75, 6WJT, 6WQK, 6WQ3, 6WVN, and 6WRZ. Plasmids have been deposited and are available from www.BEIresources.com. Raw x-ray diffraction data for small unit cell and large unit cell crystals are deposited at www.proteindiffraction.org. All other data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. This work is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/. This license does not apply to figures/photos/artwork or other content included in the article that is credited to a third party; obtain authorization from the rights holder before using such material. |
| You are subscribed to email updates from "what can cause respiratory distress,what causes sars,what is acute respiratory infection" - Google News. To stop receiving these emails, you may unsubscribe now. | Email delivery powered by Google |
| Google, 1600 Amphitheatre Parkway, Mountain View, CA 94043, United States | |
.jpg)





Comments
Post a Comment