“FDA Approves New Drug for Cutaneous T-Cell Lymphomas - Medscape” plus 1 more
“FDA Approves New Drug for Cutaneous T-Cell Lymphomas - Medscape” plus 1 more |
| FDA Approves New Drug for Cutaneous T-Cell Lymphomas - Medscape Posted: 08 Aug 2018 12:00 AM PDT 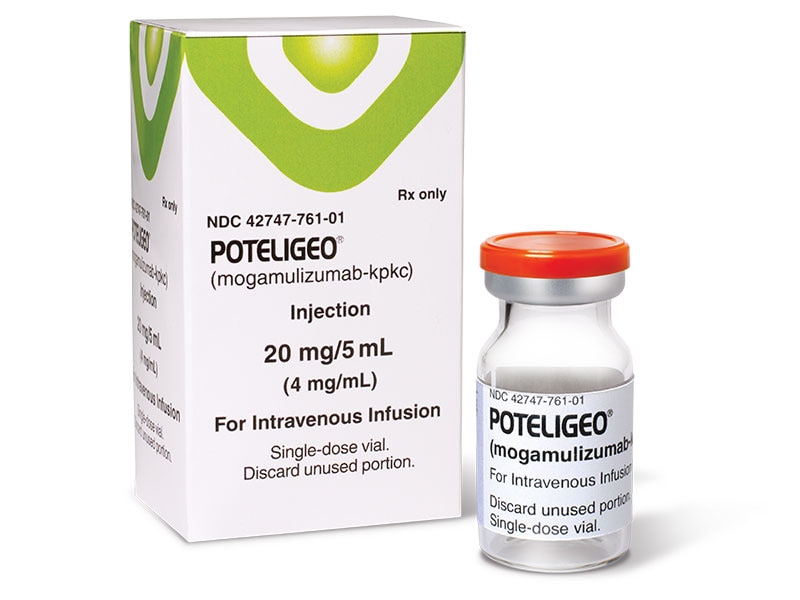 The US Food and Drug Administration (FDA) has approved mogamulizumab-kpkc (Poteligeo, Kyowa Kirin, Inc) for use in adult patients with either of two types of cutaneous T-cell lymphoma (CTCL). The drug offers a new treatment option for patients with relapsed or refractory mycosis fungoides (MF) and is the first drug to be approved for use in the treatment of Sézary syndrome (SS). MF and SS are the most common subtypes of CTCL. MF is a mature T-cell non-Hodgkin lymphoma that presents on the skin; it is the most common subtype, accounting for 50% to 70% of cases. It is a slowly progressing form of lymphoma that can involve the skin, blood, lymph nodes, and organs and may be associated with severe infections. SS is less common and accounts for approximately 3% of CTCL cases; it is a more aggressive, leukemic form of CTCL. "Mycosis fungoides and Sézary syndrome are rare, hard-to-treat types of non-Hodgkin lymphoma, and this approval fills an unmet medical need for these patients," said Richard Pazdur, MD, director of the FDA's Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA's Center for Drug Evaluation and Research, in a statement. "We are committed to continuing to expedite the development and review of this type of targeted therapy that offers meaningful treatments for patients." Mogamulizumab is a humanized monoclonal antibody directed against CC chemokine receptor 4, which is frequently expressed on leukemic cells of certain hematologic malignancies, including CTCL. It has already been approved in Japan for another rare disease, adult T-cell leukemia lymphoma. The FDA based its approval on the results from the large phase 3 MAVORIC trial. That trial compared mogamulizumab with vorinostat (Zolinza, Merck & Co), which is an FDA-approved standard-of-care treatment for patients with CTCL. The study was conducted in the United States, Europe, Japan, and Australia. A total of 372 patients with MF and SS were randomly assigned to receive either mogamulizumab or vorinostat. The results showed that mogamulizumab demonstrated significantly superior progression-free survival (PFS) with a median of 7.6 months (95% confidence interval [CI], 5.6 - 10.2), vs 3.1 months with vorinostat (95% CI, 2.8 - 4.0; hazard ratio, 0.53; 95% CI, 0.41 - 0.69; P < .001). The confirmed overall response rate was 28% with mogamulizumab vs 5% with vorinostat (P < .001). The results of the study were initially presented in 2017 at the annual meeting of the American Society of Hematology and were reported by Medscape Medical News at that time. "This is the first report of a randomized phase 3 study evaluating PFS as a primary endpoint in CTCL to compare a new systemic therapy against an FDA-approved agent, utilizing the consensus comprehensive global response criteria," said lead author Youn H. Kim, MD, professor of dermatology and director of the Multidisciplinary Cutaneous Lymphoma Program at the Stanford University School of Medicine in California when the results were presented. "Mogamulizumab demonstrated significantly superior efficacy outcomes compared to vorinostat in patients with previously treated CTCL." The most common adverse reactions (reported in ≥10% of patients) were rash, including drug eruption (35%), infusion reaction (33%), fatigue (31%), diarrhea (28%), drug eruption (24%), upper respiratory tract infection (22%), musculoskeletal pain (22%), skin infection (19%), pyrexia (17%), edema (16%), nausea (16%), headache (14%), thrombocytopenia (14%), constipation (13%), anemia (12%), mucositis (12%), cough (11%), and hypertension (10%). The FDA has noted that serious warnings regarding treatment with this agent include the risk for dermatologic toxicity, infusion reactions, infections, autoimmune problems, and complications of allogeneic stem cell transplant. Mogamulizumab received priority review, breakthrough therapy designation, and orphan drug designation. | ||||||||||||||||||||||||||||||||||||
| Posted: 31 Oct 2019 12:00 AM PDT STAINES-UPON-THAMES, United Kingdom, Oct. 31, 2019 /PRNewswire/ -- Mallinckrodt plc (NYSE: MNK), a global biopharmaceutical company, today announced that UVADEX® (methoxsalen) has received regulatory approval in Australia by the Therapeutic Goods Administration (TGA) for extracorporeal administration with the THERAKOS® CELLEX® Photopheresis System. The treatment is indicated for steroid-refractory and steroid-intolerant chronic graft versus host disease (cGvHD) in adults following allogeneic hematopoietic stem cell (HSC) transplantation. The TGA also approved Uvadex in conjunction with the THERAKOS CELLEX Photopheresis System for the palliative treatment of skin manifestations of cutaneous T-cell lymphoma (CTCL) that is unresponsive to other forms of treatment. The TGA approval marks the first combined indication label and the first regulatory approval in the world for UVADEX in conjunction with the THERAKOS Photopheresis System for the treatment of chronic graft versus host disease in adults. "The TGA approval of UVADEX with the Therakos ECP platform opens up new treatment options for patients with these challenging conditions," said Steven Romano, M.D., Executive Vice President and Chief Scientific Officer, Mallinckrodt. "The cGvHD indication is also an important milestone for Mallinckrodt, confirming the potential benefit of this therapeutic option for patients who are refractory to or intolerant of steroid treatments." About Chronic Graft Versus Host Disease (cGvHD) About Cutaneous T-Cell Lymphoma (CTCL) Minimum Product Information: UVADEX® (methoxsalen) Concentrated Injection for extracorporeal circulation via photopheresis (ECP) This medicinal product is subject to additional monitoring in Australia. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse events at www.tga.gov.au/reporting-problems. Indications in Australia: UVADEX (methoxsalen) is indicated for extracorporeal administration with the THERAKOS CELLEX Photopheresis System for the:
Contraindications: History of idiosyncratic or hypersensitivity reaction to methoxsalen, psoralen compounds or any excipients of UVADEX; co‑existing melanoma, basal cell or squamous cell skin carcinoma; lactation; aphakia. ECP procedure contra-indications: Photosensitive disease; inability to tolerate extracorporeal volume loss; WBC count > 25,000 mm3; previous splenectomy; coagulation disorders. Special warnings and precautions: Only physicians who have special competence in the diagnosis and treatment of cGVHD and CTCL who have special training and experience with the THERAKOS CELLEX Photopheresis System should use UVADEX. Men and women being treated with UVADEX should take adequate contraceptive precautions both during and after completion of photopheresis treatment. Exposure to large doses of UVA causes cataracts in animals, an effect enhanced by the administration of oral methoxsalen. The patient's eyes should be protected from UVA light by wearing wrap‑around, UVA‑opaque sunglasses during the treatment cycle and during the following 24 hours. Exposure to sunlight or ultraviolet radiation (even through window glass) may result in serious burns and, in the long‑term, "premature aging" of the skin therefore patients should avoid exposure to sunlight during the 24 hours following photopheresis treatment. Thromboembolic events, such as pulmonary embolism and deep vein thrombosis, have been reported with UVADEX administration through photopheresis systems for treatment of patients with graft versus host disease. This product contains 4.1% w/v ethanol and each 1 mL of UVADEX contains 40.55 mg of ethanol. Caution is advised in patients with liver disease, alcoholism, epilepsy, brain injury or disease. No specific information is available for use in renal or hepatic impairment and there is no evidence for dose adjustment in the elderly. The safety and efficacy of UVADEX have not been established in children. Use in pregnancy: Category D. Use in Lactation: UVADEX is contra-indicated. Interactions with other medicines: Effects on P450 system metabolism may affect clearance / activation of other drugs (caffeine, paracetamol) or may extend the methoxsalen half-life leading to prolonged photosensitivity in patients. Methoxsalen binding to albumin may be displaced by dicoumarol, warfarin, promethazine and tolbutamide with potential for enhanced photosensitivity. Caution when treating with concomitant photosensitising agents. Adverse effects: In the clinical trials, published information and postmarketing surveillance of UVADEX/ECP, adverse events were usually mild and transient and in most cases, related to the underlying pathology. Very common: diarrhoea, anaemia, nausea, headache, hypertension, sinusitis, upper respiratory tract infection, fatigue, pain in extremity, pyrexia, cough, dyspnoea, cushingoid, dry eye, photophobia, toothache, anorexia. Common: depression, lacrimation increased, abdominal pain, hypokalaemia, paraesthesia oral, pharyngolaryngeal pain, tachycardia, conjunctivitis, eye pain, visual acuity reduced, dysphagia, chills, mucosal inflammation, nasopharyngitis, contusion, blood pressure diastolic decreased, haemoglobin decreased, hyperglycaemia, hypocalcaemia, neuropathy peripheral, tremor, rash, hypotension. Additional adverse effects seen in clinical trials include vomiting, infections. Adverse events related to the ECP/CELLEX procedure – thromboembolism and severe allergic reactions, vascular access complication, vasovagal spasm, hickman catheter infection/thrombosis, headache, hypercoagulability, haemolysis. Additional adverse events identified post-marketing: anaphylactic reaction, allergic reaction, dysgeusia, exacerbation of congestive heart failure, sepsis, endocarditis, and vomiting. Dosage and Administration: Chronic Graft versus Host Disease: Three ECP treatments in the first week then two ECP treatments per week for at least 12 weeks, or as clinically indicated. Cutaneous T-cell Lymphoma: ECP treatment on two successive days each month for six months. Patients who show an increase in skin scores after eight treatment sessions may have their treatment schedule increased to two successive days every two weeks for the next three months. Refer to full Product Information and THERAKOS CELLEX Operator's Manual for information regarding administration. Store below 25°C. Date of first approval: 16 September 2019. Date of revision: 11 October 2019. Indications and Prescribing Information for Uvadex vary globally. Please refer to the individual country product label for complete information. Before prescribing Uvadex, please refer to the full Product Information also available by calling + 1 800.778.7898. ABOUT THERAKOS Terumo BCT is the exclusive distributor of the Therakos ECP platform in Australia, as well as Latin America and select countries in Europe. To learn more about Terumo BCT, visit www.terumobct.com. UVADEX (methoxsalen) and THERAKOS CELLEX Photopheresis Systems are separately approved in a number of global markets. Please refer to your local approved labelling for Uvadex and the Operator's Manual for CELLEX for more information on approved uses for specific indications. Before administering therapy using the THERAKOS CELLEX Photopheresis System, please refer to the Operator's Manual available at +61 2 9429 3600 or +1 (800)778-7898. ABOUT MALLINCKRODT Mallinckrodt uses its website as a channel of distribution of important company information, such as press releases, investor presentations and other financial information. It also uses its website to expedite public access to time-critical information regarding the company in advance of or in lieu of distributing a press release or a filing with the U.S. Securities and Exchange Commission (SEC) disclosing the same information. Therefore, investors should look to the Investor Relations page of the website for important and time-critical information. Visitors to the website can also register to receive automatic e-mail and other notifications alerting them when new information is made available on the Investor Relations page of the website. CAUTIONARY STATEMENTS RELATED TO FORWARD-LOOKING STATEMENTS CONTACTS Investor Relations Mallinckrodt, the "M" brand mark and the Mallinckrodt Pharmaceuticals logo are trademarks of a Mallinckrodt company.
SOURCE Mallinckrodt Pharmaceuticals  Related Links | ||||||||||||||||||||||||||||||||||||
| You are subscribed to email updates from "severe respiratory distress,severe respiratory infection,sezary syndrome" - Google News. To stop receiving these emails, you may unsubscribe now. | Email delivery powered by Google |
| Google, 1600 Amphitheatre Parkway, Mountain View, CA 94043, United States | |
Comments
Post a Comment